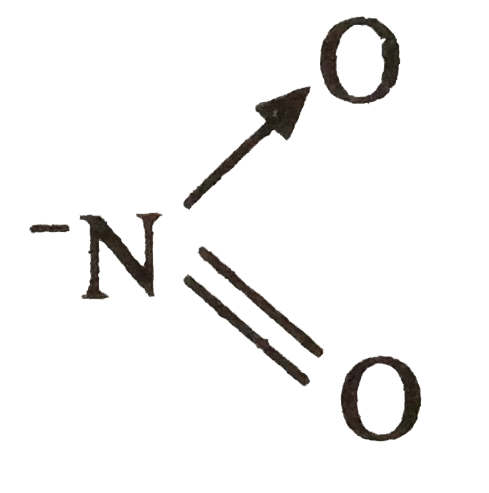
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQS|84 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise COMPREHENNSION TYPE MCQS|5 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise Brain storming multiple choice question|12 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -REVISION QUESTIONS
- A similarity between optical and geometrical isomerism is that
Text Solution
|
- A square planar complex is formed by hybridization of which atomic orb...
Text Solution
|
- The type of isomerism present in intro pentaamine-chromium(III)chlorid...
Text Solution
|
- Which of the following compounds is square planar and does not have an...
Text Solution
|
- Which of the following hydrate is diamagnetic ?
Text Solution
|
- The geometry of Ni(CO(4)) and Ni(PPh(3))(2)Cl(2) are
Text Solution
|
- Hybridization of Fe in K(3)Fe(CN)(6) is
Text Solution
|
- One mole of complex compound Co(NH3)5Cl3 gives 3 moles of ions on diss...
Text Solution
|
- Ammonia forms the complex [Cu(NH3)4]^(2+) with copper ions in alkaline...
Text Solution
|
- Which one of following octahedral complexes will not show geometrical ...
Text Solution
|
- The number of unpaired electrons in the complex ion [CoF6]^(3-) is (At...
Text Solution
|
- According to IUPAC nomenclature sodium nitroprusside is named as
Text Solution
|
- In the coordination compound, K4[Ni(CN)4] oxidation state of nickel is
Text Solution
|
- The complex used as an anticancer agent is
Text Solution
|
- The correct name of the compound [Cu(NH(3))(4)](NO(3))(2), according t...
Text Solution
|
- Which of the following complex species does not involve inner orbital ...
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Which one of the following complexes? [Atomic numbers, Mn=25, Fe = 26,...
Text Solution
|
- The coordination number of a central metal atom in a complex is determ...
Text Solution
|
- CN^(-) is a strong field ligand. This is due to the fact that
Text Solution
|