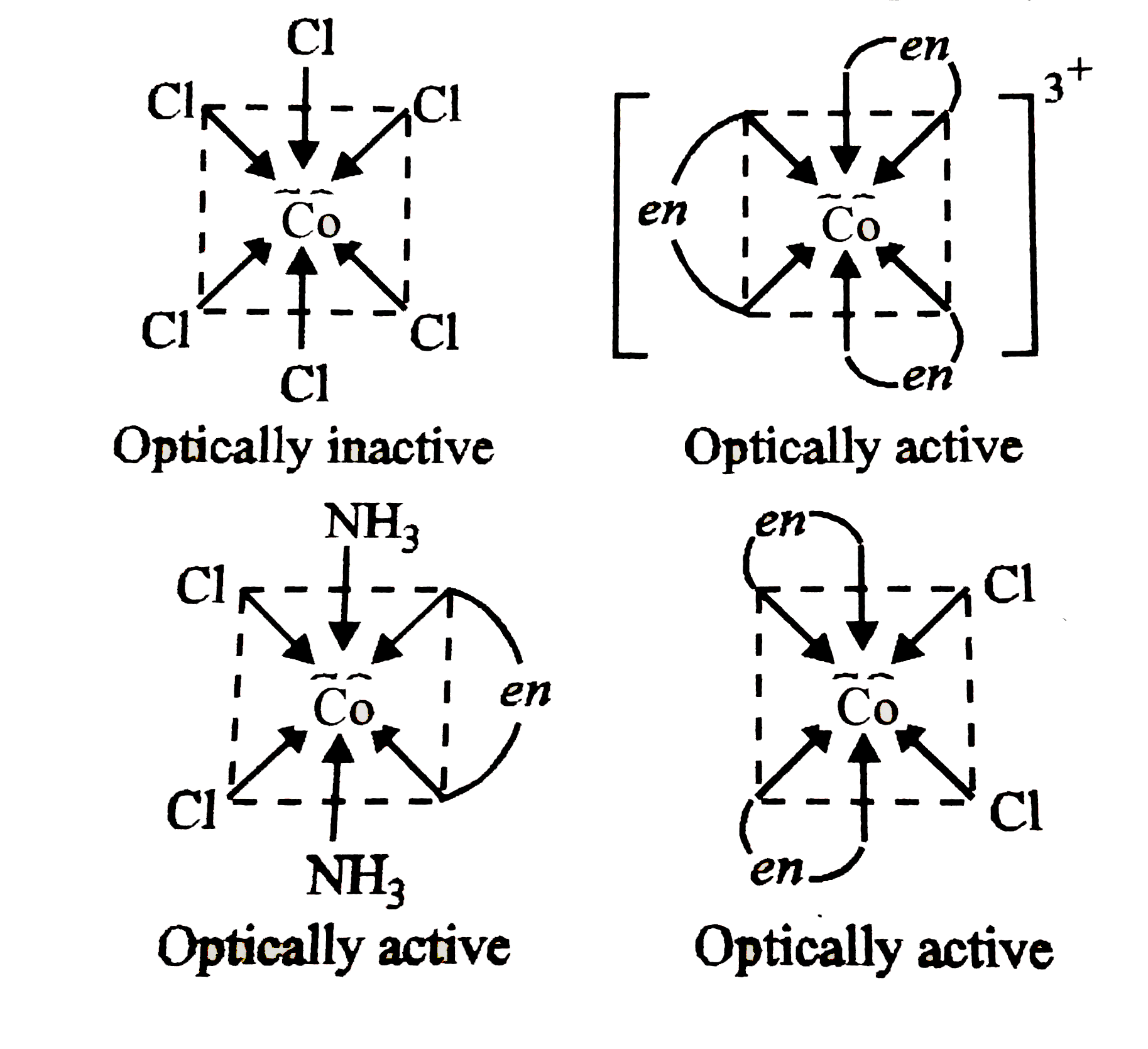A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQS|84 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise COMPREHENNSION TYPE MCQS|5 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise Brain storming multiple choice question|12 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -REVISION QUESTIONS
- Considering H2O as a weak field ligand, the number of unpaired electro...
Text Solution
|
- Which of the following is not considered as an organometallic compound...
Text Solution
|
- Which of the following does not have optical isomer?
Text Solution
|
- Pentaamminenitrocobalt (III) cation possesses the property of
Text Solution
|
- The anion [Co(C(2)O(4))(3)]^(3-) involves hybridization
Text Solution
|
- Match the list I and II and pick the correct matching from the codes g...
Text Solution
|
- The number for chloride ion/s produced by complex tetraamminechloropla...
Text Solution
|
- The prussian blue colour obtained during the test of nitrogen by lassa...
Text Solution
|
- The oxidation state of Cr in [Cr(NH(3))(4)Cl(2)]^(+) is:
Text Solution
|
- [Fe(NO(2))(3)Cl(3)] and [Fe(O-NO)(3)Cl(3)] show
Text Solution
|
- In which of the following complex ion, the central metal ions is in a ...
Text Solution
|
- Which of the following has highest molar conductivity
Text Solution
|
- Match List-I (Complexes) with List-II (Hybridization) of central atom ...
Text Solution
|
- When excess of ammonia is added to CuSO4 solution, the deep blue colou...
Text Solution
|
- Among the following complexes which has magnetic moment of 5.9 BM
Text Solution
|
- The formula of sodium nitroprusside is
Text Solution
|
- For mu isomerism is associated with which one of the following complex...
Text Solution
|
- The increasing order of the crystal field splitting power of some comm...
Text Solution
|
- Which of the following is a tridentate ligand?
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|