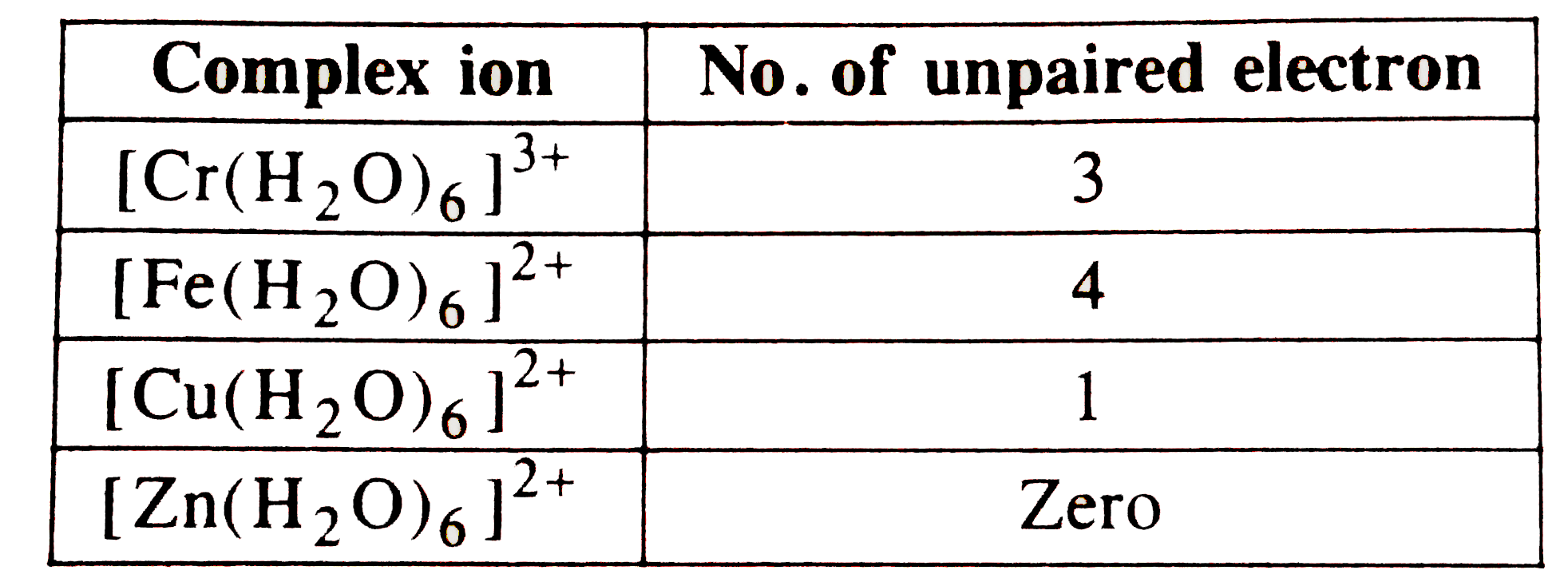
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise COMPREHENNSION TYPE MCQS|5 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise MATRIX MATCH TYPE MCQS|3 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise REVISION QUESTIONS|165 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -SELECTED STRAIGHT OBJECTIVE TYPE MCQS
- Which of the following are paramagnetic?
Text Solution
|
- Which of the following statements about [Fe(H(2)O)(5)NO]^(2-) are corr...
Text Solution
|
- Select the complexes in which iron is in +2 oxidation state ?
Text Solution
|
- K(4)[Fe(CN)(6)] is used for detection of
Text Solution
|
- K(3)[Fe(CN)(6)] is used for the detection of
Text Solution
|
- A ligand can be
Text Solution
|
- The oxidation state of Fe in brown complex [Fe(H(2)O)(5)NO]SO(4) is
Text Solution
|
- Amongst Ni(CO)(4),[Ni(CN)(4)]^(2-) and [NiCl(4)]^(2-)
Text Solution
|
- The compound which does not show paramagnetism is
Text Solution
|
- The number of d-electrons in [Cr(H(2)O)(6)]^(3+) [At. No. of Cr = 24 ]...
Text Solution
|
- Among the following ions, which one has the highest paramagnetism?
Text Solution
|
- Which of the following is an organometallic compound ?
Text Solution
|
- In nitroprusside ion, the iron and NO exist as Fe(II) and NO^(+) rathe...
Text Solution
|
- The geometry of Ni(CO(4)) and Ni(PPh(3))(2)Cl(2) are
Text Solution
|
- The pair having the same magnetic moment is [at. No. Cr = 24, Mn = ...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- A mixture x containing 0.02 mol of [Co(NH(3))(5) SO(4)]Br and 0.02 mol...
Text Solution
|
- In the process of extraction of gold. Roasted gold ore +CN^(-)+H(2)O...
Text Solution
|
- The pair of the compounds in which both the metals are in the highest ...
Text Solution
|
- The species having tetrahedral shape is
Text Solution
|