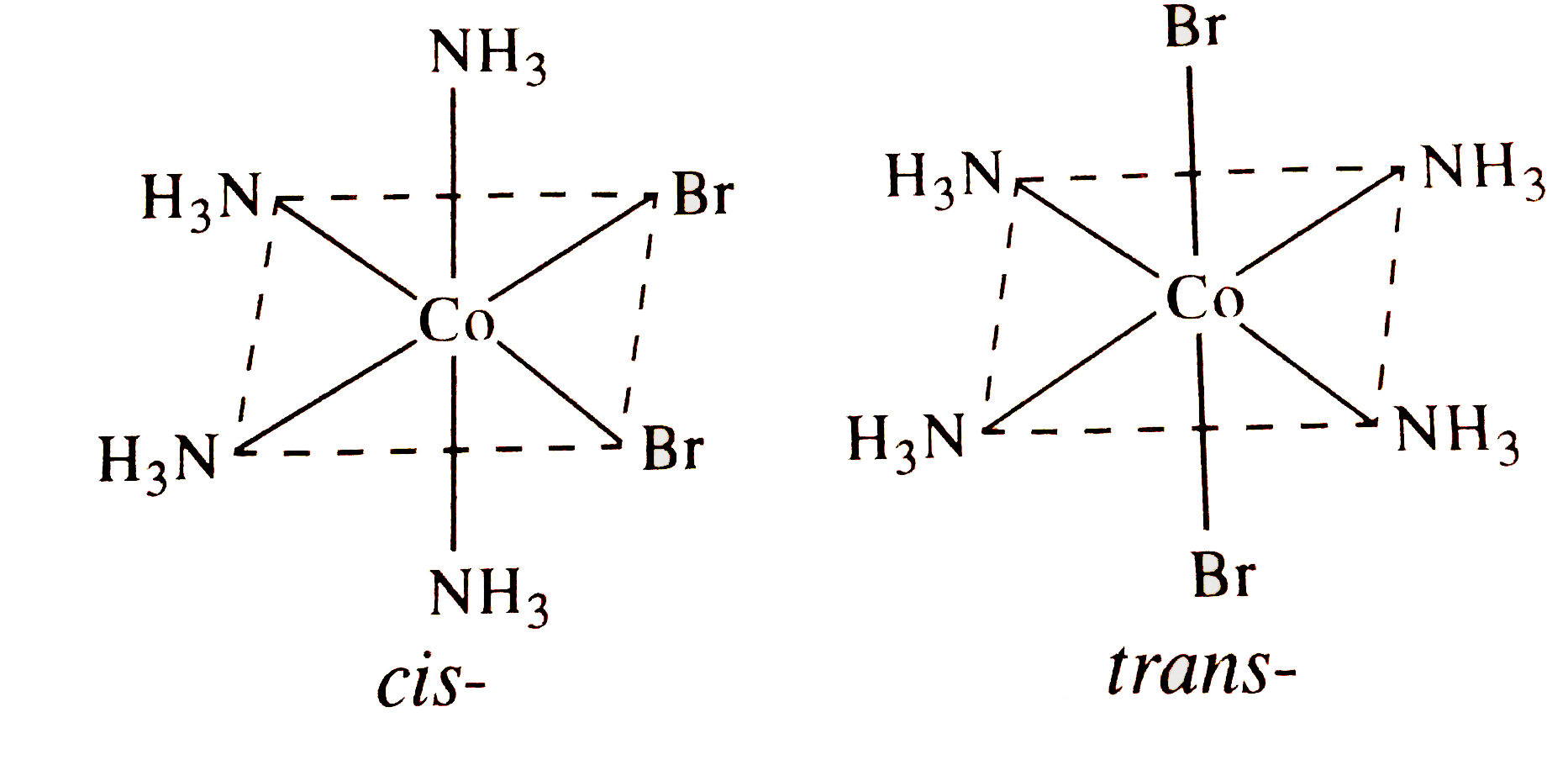A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise COMPREHENNSION TYPE MCQS|5 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise MATRIX MATCH TYPE MCQS|3 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise REVISION QUESTIONS|165 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -SELECTED STRAIGHT OBJECTIVE TYPE MCQS
- The pair of the compounds in which both the metals are in the highest ...
Text Solution
|
- The species having tetrahedral shape is
Text Solution
|
- Which kind of isomerism is shown by Co (NH(3))(4) Br(2) Cl ?
Text Solution
|
- In which of the following pairs both the complexes do not show optical...
Text Solution
|
- The diamagnetic species is
Text Solution
|
- The correct order for the wavelength of absorption in the visible regi...
Text Solution
|
- Which of the following compounds show optical isomerism?
Text Solution
|
- Which one of the cyano complexes would exhibit the lowest value of par...
Text Solution
|
- Which of the following is an inner orbital complex as well as diamagne...
Text Solution
|
- Which one of the following is expected to exhibit optical isomerism (e...
Text Solution
|
- IUPAC name [Co(NH(3))(5)(NO(2))]Cl(2) is
Text Solution
|
- Nickel (Z=28) combines with a uninegative monodentate ligand X^(-) to ...
Text Solution
|
- In Fe(CO)5, the Fe-C bond possesses:
Text Solution
|
- How many EDTA molecules are required to make an octahedral complex wit...
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- [Co(NH(3))(4)(NO(2))(2)]CI exhibits
Text Solution
|
- Copper sulphate dissolves in excess of KCN to give
Text Solution
|
- The pair in which both species have same magnetic moment (spin only va...
Text Solution
|
- The number of possible of an octahedral complex [Co(C(2)O(4))(2)(NH(3)...
Text Solution
|
- Among the following , the species having square planar geometry for ce...
Text Solution
|