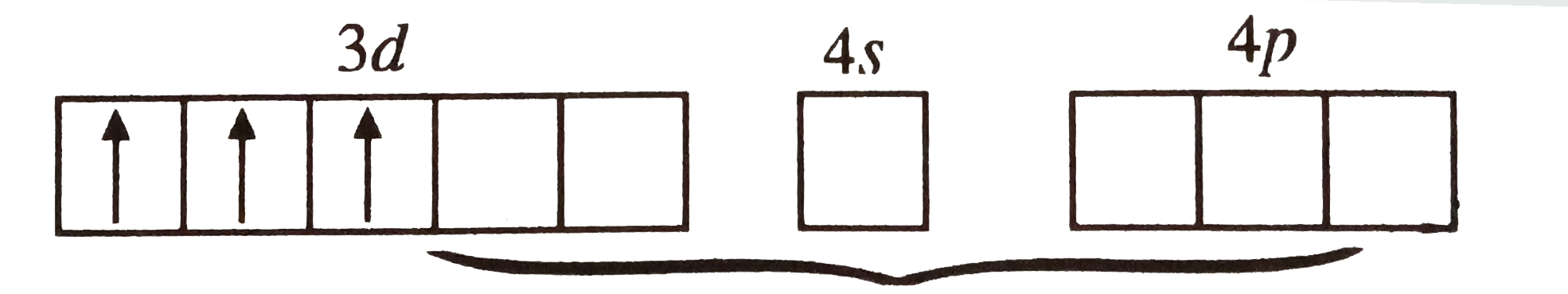
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise COMPREHENNSION TYPE MCQS|5 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise MATRIX MATCH TYPE MCQS|3 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise REVISION QUESTIONS|165 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -SELECTED STRAIGHT OBJECTIVE TYPE MCQS
- In Fe(CO)5, the Fe-C bond possesses:
Text Solution
|
- How many EDTA molecules are required to make an octahedral complex wit...
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- [Co(NH(3))(4)(NO(2))(2)]CI exhibits
Text Solution
|
- Copper sulphate dissolves in excess of KCN to give
Text Solution
|
- The pair in which both species have same magnetic moment (spin only va...
Text Solution
|
- The number of possible of an octahedral complex [Co(C(2)O(4))(2)(NH(3)...
Text Solution
|
- Among the following , the species having square planar geometry for ce...
Text Solution
|
- Ag^(+)+hArr|Ag(NH(3))(2)|^(+), k(1) = 6.8 xx 10^(-3) [Ag(NH(3))]^(+)...
Text Solution
|
- If the bond length of CO bond in carbon monoxide is 1.128 Å, then what...
Text Solution
|
- The d electron congfiguration of Cr^(2+) , Mn^(2+) , Fe^(2+) and Ni^(2...
Text Solution
|
- Which of the following has a square planar geometry? .
Text Solution
|
- Among the following metal carbonyls, the C-O bond order is lowest in
Text Solution
|
- Which of the following will give a pair of enontiomorphs ? en = NH(2...
Text Solution
|
- The IUPAC name of [Ni (NH(3))(4)] [NiCl(4)] is
Text Solution
|
- Which of the following complexes exhibits the highest paramagnetic beh...
Text Solution
|
- In which of the following coordination entities the magnitude of Delta...
Text Solution
|
- The coordination number and the oxidation state of the element 'E' in ...
Text Solution
|
- For the given complex [CoCl(2)(en)(N(3))(2)]^(+), the number of geomet...
Text Solution
|
- The spin only magnetic moment value of Cr(CO)(6) is
Text Solution
|