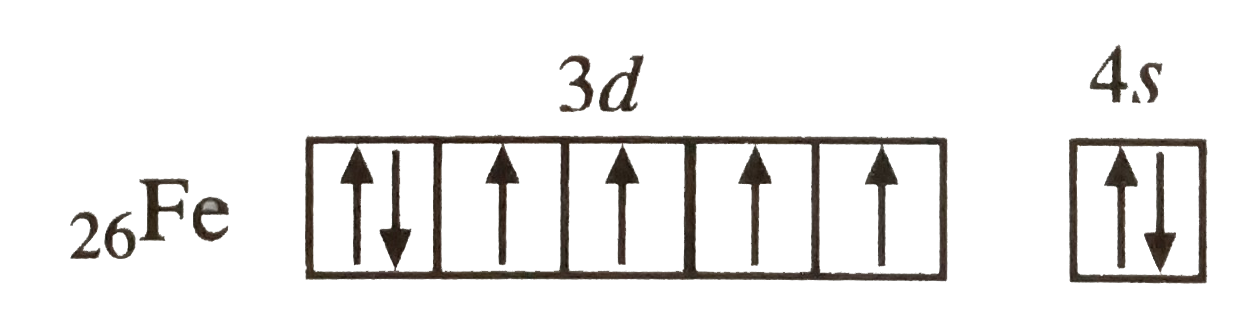
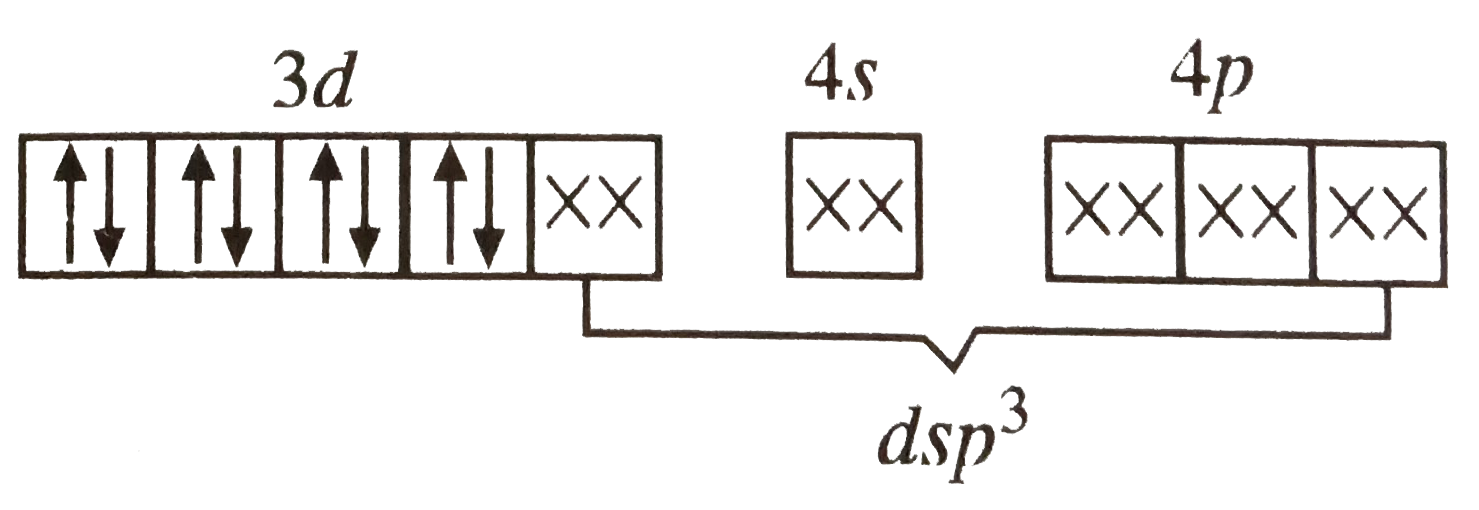
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise COMPREHENNSION TYPE MCQS|5 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise MATRIX MATCH TYPE MCQS|3 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise REVISION QUESTIONS|165 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -SELECTED STRAIGHT OBJECTIVE TYPE MCQS
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- Which of the following is diamagnetic in nature ?
Text Solution
|
- In Fe(CO)(5), the Fe larr CO sigma bond results by the overlap between...
Text Solution
|
- The ligand N(CH(2)CH(2)NH(2))(3) is
Text Solution
|
- Which one of the following has largest number of isomers?
Text Solution
|
- Square planar complexes of the type MABXL (where A, B, X and L are uni...
Text Solution
|
- Which of the following complex ions has geometrical isomers?
Text Solution
|
- Which 0.01 mole of a cobalt complex is treated with excess silver nitr...
Text Solution
|
- The correct statement with respect to the complexes Ni(CO)(4) and |Ni(...
Text Solution
|
- The complex which has the highest magnetic moment among the following ...
Text Solution
|
- Among |Ni(CO)(4)|, |Ni(CN)(4)|^(2-), |NiCl(4)|^(2-) species, the hybri...
Text Solution
|
- Geometrical shapes of the complex formed by the reaction of Ni^(2+) wi...
Text Solution
|
- Among the following complexes : K(3)[Fe(CN)(6)],[Co(NH(3))(6)]Cl(3) , ...
Text Solution
|
- Which of the following facls about the complex |Cr(NH(3))(6)|Cl(3) is ...
Text Solution
|
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- [NiCl(2){P(C(2)H(5))(2)(C(6)H(5))}(2)] exhibits temperature dependent ...
Text Solution
|
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- As per IUPAC nomenclature, the name of the complex [Co(H(2)O)(4) (NH(3...
Text Solution
|
- Which of the following complex species is not expected to exhibit opti...
Text Solution
|
- An excess of AgNO(3) is added to 100mL of a 0.01M solution of dichloro...
Text Solution
|