Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Concept based questions|19 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise H.O.T.S. Conceptual Questions|11 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Board Examinations|67 VideosSTATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Statement Type Question|5 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise Ultimate Prepatarory Package|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STRUCTURE OF ATOM-Short Answer type Questions
- Calculate the total number of angular nodes and radical nodes present ...
Text Solution
|
- The arrangement of orbitals on the basis of energy is based upon their...
Text Solution
|
- Which of the following will not show deflection from the path on passi...
Text Solution
|
- An atom having mass number 13 has 7 neutrons. What is the atomic numbe...
Text Solution
|
- Wavelengths of different radiations are given below: (A) 300nm (B) ...
Text Solution
|
- The electronic configuration of valence shell of Cu is 3d^(10)4s^(2). ...
Text Solution
|
- The Balmer series in the hydrogen spectrum corresponds to the transiti...
Text Solution
|
- According to de-Brogile, matter should exhibit dual behaviour, that i...
Text Solution
|
- What is the experimental evidence in support of the diea that electron...
Text Solution
|
- Out of electron and proton which one will have, a higher velocity to p...
Text Solution
|
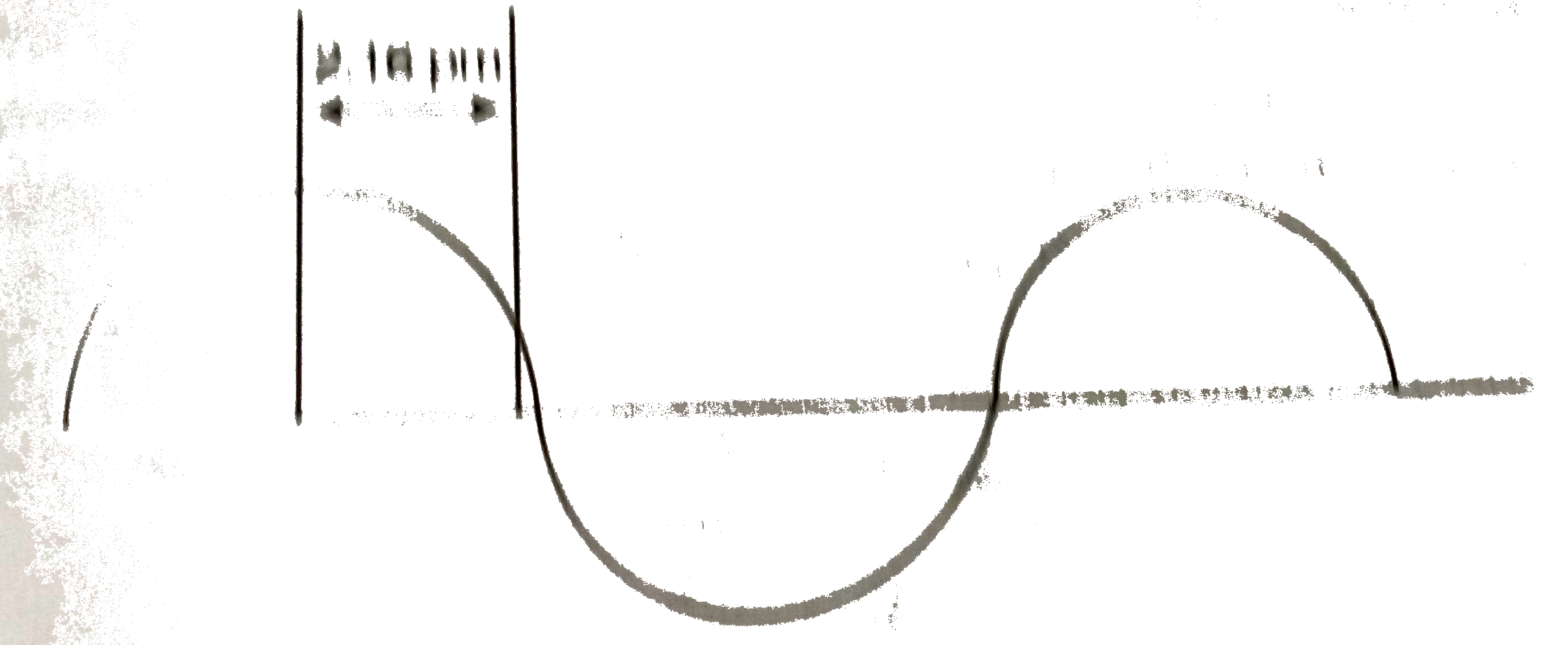
- A hypothetical electromagnetic wave is shown in figure. Find out the w...
Text Solution
|
- Chlorophyll present in green leaves of plants absorbs light at 4.620xx...
Text Solution
|
- What is the difference between the terms orbit and orbital ?
Text Solution
|
- Table-tennis ball has mass 10g and s peed of 90m/s. if speed can be me...
Text Solution
|
- The effect of uncertainty principle is significant only for motion of ...
Text Solution
|
- Hydrogen atom has only one electron, So, mutual repulsion between elec...
Text Solution
|
- Thershold frequency, v(0) is the minimum frequency which a photon must...
Text Solution
|
- When an electric discharge is passed through hydrogen gas, the hydroge...
Text Solution
|
- Calculate the energy and frequency of the radiation emitted when an el...
Text Solution
|
- Why was a change in the Bohr Model of atom required ? Due to which imp...
Text Solution
|