Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
DINESH PUBLICATION|Exercise QUESTION FROM BOARD EXAMINATIONS|110 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONA AND NUMERICAL PROBLEMS|11 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS|4 VideosSOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|20 VideosTHE CARBON FAMILY
DINESH PUBLICATION|Exercise Ultimate|11 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SURFACE CHEMISTRY -ADDITIONAL IMPORTANT QUESTIONS
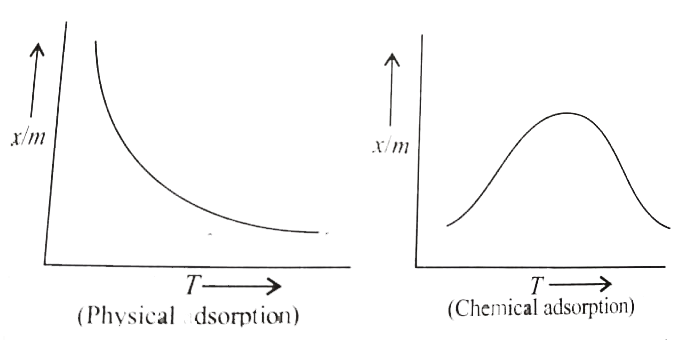
- Physical and chemical adsorption respond differently with a rise in te...
Text Solution
|
- Identify the dispersed phase and dispersion medium in the following ex...
Text Solution
|
- For drying H(2)S gas concentrated H(2)SO(4) can not be used why ?
Text Solution
|
- When freshly precipitated Fe(OH)(3) is shaken with aqueous solution of...
Text Solution
|
- A difference between diffusion and osmosis is
Text Solution
|
- 2-56 g of sulphur present in 100 mL of solution in colloidal form show...
Text Solution
|
- Why does a secondary rainbow have inverted colours ?
Text Solution
|
- on adding AgNO(3) solution into KI solution , a negatively charged col...
Text Solution
|
- The extent of adsorption of a gas depends upon the
Text Solution
|
- Which of the following gases can be liquefied easily ?
Text Solution
|
- The critical temperature of O(2) and N(2) are 155K and 126 K respecti...
Text Solution
|
- Gelatin is generally added to ice creams. Why?
Text Solution
|
- Which one of the following acts as the best coagulating agent for ferr...
Text Solution
|
- Lyophilic sols are more stable than lyophobic sols because their parti...
Text Solution
|
- Artificial rain can be caused by spraying charged dust particles over ...
Text Solution
|
- Ferric hydroxide sol is more readily conagulated by Na(3)PO(4) in comp...
Text Solution
|
- Delta is generally formed wheere river meets the ocean. How will you a...
Text Solution
|
- The layer of fat in the pans used for manufacturing soaps can be remov...
Text Solution
|
- Which out of the following solution having the same concentration will...
Text Solution
|
- 100 mL of a standard sol required 240 mg of starch for its protection ...
Text Solution
|