Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
DINESH PUBLICATION|Exercise MULTIPLE CHOICE QUESTION BANK (MCQB)|28 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise JEE (MAIN) & OTHER ENGINEERING ENTRANCE EXAMINATIONS|33 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise QUESTION FROM BOARD EXAMINATIONS|110 VideosSOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|20 VideosTHE CARBON FAMILY
DINESH PUBLICATION|Exercise Ultimate|11 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SURFACE CHEMISTRY -HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONA AND NUMERICAL PROBLEMS
- In order to coagulate a fixed amount of As(2)S(3), sol, how will NaCl...
Text Solution
|
- A student wrote the following explanation about the working of a catal...
Text Solution
|
- In the Freundlich adsorption isotherm, the value of x/m is 0.4 under a...
Text Solution
|
- Graph between log x/m and log P is a straight line at angle of 45^(@) ...
Text Solution
|
- Consider the adsorption isotherm given below and interpret the variati...
Text Solution
|
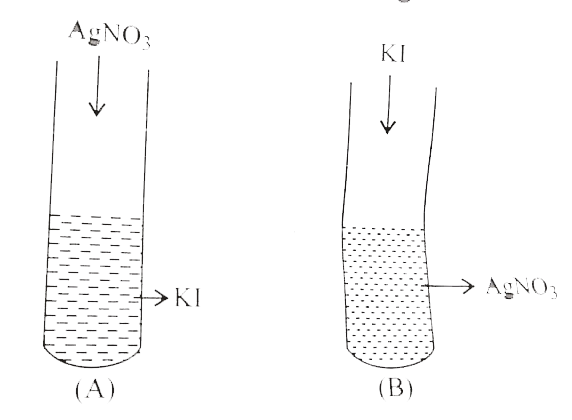
- A colloidal solution of Agl is prepared by two different methods as sh...
Text Solution
|
- 100 mL of 0.3 M acetic acid is shaken with 0.8 g wood charcoal. The fi...
Text Solution
|
- In the adsorption of acetic acid vapours by 1 g of charcoal, the follo...
Text Solution
|
- SnO(2) form a positively charged colloidal sol in the acidic medium an...
Text Solution
|
- When bezoyl chloride si reduced by H(2) in the presence of Pd catalyst...
Text Solution
|
- To the aqueous solution of a slat taken in a tube, a feq drops of blue...
Text Solution
|