A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
DINESH PUBLICATION|Exercise ASSERTIN-REASON TYPE QUESTIONS|25 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQs)|8 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise COMPRHENSION LINKED MCQs|39 VideosSOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|20 VideosTHE CARBON FAMILY
DINESH PUBLICATION|Exercise Ultimate|11 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SURFACE CHEMISTRY -MULTIPLE CORRECT OPTIONS TYPE MCWs
- Which of the following are lyophilic in nature ?
Text Solution
|
- Select the correct statement among the following:
Text Solution
|
- Which fo the following statements are ture ?
Text Solution
|
- Which of following are examples of aerosols ?
Text Solution
|
- Which of the following are macromolecular colloids ?
Text Solution
|
- Methods for preparing colloidal sols are :
Text Solution
|
- Multimolecular colloids are present in :
Text Solution
|
- Which among the following are enzyme catalysts ?
Text Solution
|
- Choose the correct reason (s) for the stability of lyophobic colloidal...
Text Solution
|
- A mol of [Ag]Ag^(+) sol cagulated by :
Text Solution
|
- Raeaction of zeolite catalyst depend upon :
Text Solution
|
- The correct statement (S) pertaining to the adsorption of a gas on a s...
Text Solution
|
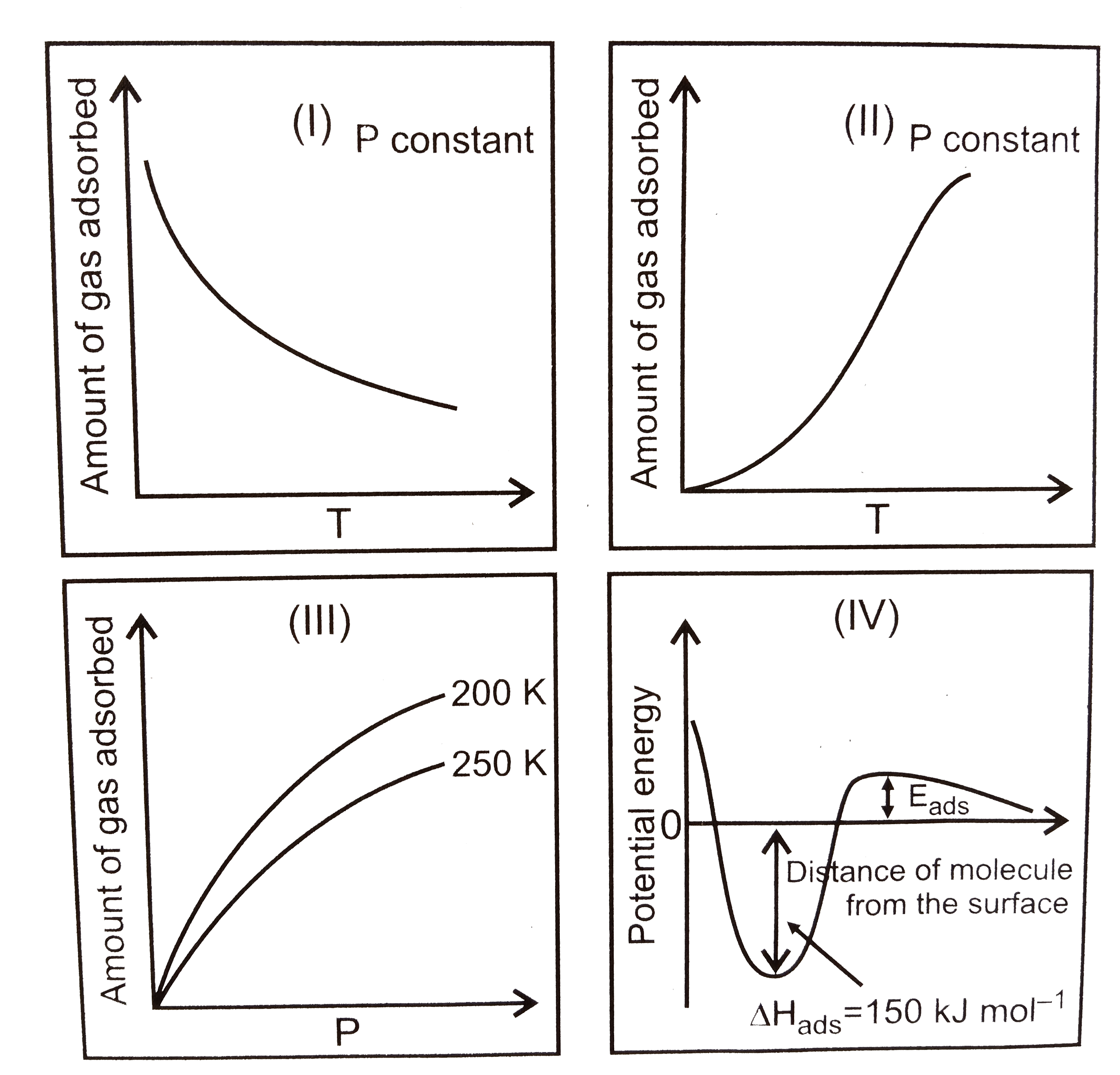
- The given graphs//data I, II, II and IV pepresent general terends ob...
Text Solution
|
- When O(2) is adsorbed on a metallic surface, electron transfer occurs...
Text Solution
|
- The correct (S) about surface properties is (are)
Text Solution
|
 .
.