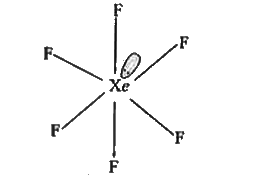
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SIA PUBLICATION-p-BLOCK ELEMENTS-MCQ.s
- What are the products formed when moist chlorine gas is reacted with h...
Text Solution
|
- What is the bond angle in ClO(2) (OClO) ?
Text Solution
|
- The hybridisation of Xe and the number of lone pairs of electrons on i...
Text Solution
|
- Diborane reacts with ammonia under different conditions to give a vari...
Text Solution
|
- Which one of the following is the mineral for tin?
Text Solution
|
- The oxide of nitrogen formed by thermal decoposition of NH(4)NO(3) is
Text Solution
|
- Which one of the following is most acidic?
Text Solution
|
- Which one of the following is formed apart from sodium chloride when c...
Text Solution
|
- Helium mixed with oxygen is used in the treatment of
Text Solution
|
- The number of p pi-d pi 'pi' bonds present in XeO(3) and XeO(4) molecu...
Text Solution
|
- The type of bonds present in sulphuric anhydride are
Text Solution
|
- Which pair of oxyacids of phosphorus contains 'P-H' bonds ?
Text Solution
|
- SiCl(4) on hydrolysis forms 'X' and HCl. Compound 'X' loses water at 1...
Text Solution
|
- Match the following The correct answer is
Text Solution
|
- Boron halides behave as Lewis acids because of their nature.
Text Solution
|
- Identify B in the following reaction H(4)SiO(4) underset(-H(2)O)overse...
Text Solution
|
- The correct oder of reducing abilities of hydrides of V group elements...
Text Solution
|
- The number of sigma and pi bonds in peroxodisulphuric acid are, respec...
Text Solution
|
- Which one of the following reactions does not occur?
Text Solution
|
- The formula of the product formed, when sodium triosulphite solution i...
Text Solution
|