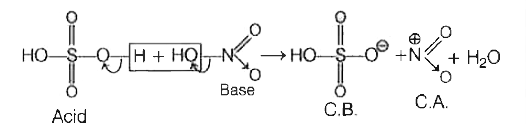A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the process of formation of nitronium ion, nitric acid acts as
Text Solution
|
- Treatment of glucose with nitric acid leads to the formation of a
Text Solution
|
- S-I: Nitration of benzene with nitric acid requires the use of concent...
Text Solution
|
- How is nitric acid prepared by Ostwald's process ?
Text Solution
|
- नाइट्रोनियम आयन (NO2^(+)) अभिकर्मक है ।
Text Solution
|
- Nitronium ion is isoelectronic with
Text Solution
|
- During nitration of benzene with a mixture of concentrated nitric acii...
Text Solution
|
- In the process of formation of nitronium ion, nitric acid acts as
Text Solution
|
- Explain the formation of nitronium ion (overset(oplus)NO2) from the re...
Text Solution
|