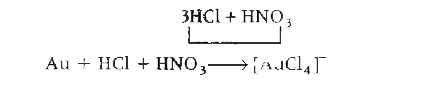A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the final chemical form of Gold (Au) when it is dissolved in a...
Text Solution
|
- Gold dissolves in aqua regia forming
Text Solution
|
- The IUPAC name of the complex ion formed when gold dissolves in aqua-r...
Text Solution
|
- Gold dissolves in a aqua-regia forming:
Text Solution
|
- What is the final chemical form of Gold (Au) when it is dissolved in a...
Text Solution
|
- What is aqua regia ? Explain why Au and Pt dissolve in aqua regain .
Text Solution
|
- Gold dissolves in aqua regia to form -
Text Solution
|
- Gold dissolves in a aqua-regia forming:
Text Solution
|
- Gold dissolves in aqua-regia forming
Text Solution
|