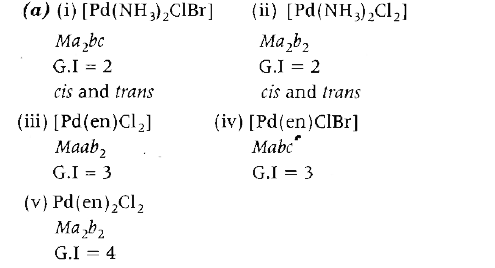A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the complexs. (i) [Pd(NH(3))(2)ClBr] [Pd(NH(3))(2)Cl(2)] ...
Text Solution
|
- The complex [Pd(en)(2)]^(2+) has....Structure
Text Solution
|
- Draw all the isomers (geometrical and optical) of: (i) [CoCl(2)(en)(...
Text Solution
|
- Total number of possible isomers of complex [Pd(NH(3))(2)(SCN)(2)]
Text Solution
|
- [Co(NH(3))(2)Cl(2)(en)]^(+) के ज्यामितीय समावयवी बनाइए।
Text Solution
|
- निम्नलिखित संकर यौगिकों के प्रकाशिक समावयवी लिखिए- (i) [Co(Cl(2))(...
Text Solution
|
- Draw all the isomers (geometricals and optical) of (i) [CoCl(2)(en)(2)...
Text Solution
|
- Number of possible isomer for the complex [Co(en)(2)CI(2)]CI will be: ...
Text Solution
|
- Among the following, total number of species is/are existing as optica...
Text Solution
|