A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET ENGINEERING ENTRANCE EXAM QUESTION PAPER (2018)-CHEMISTRY
- The correct option for the first ionisation enthalpy (in kJ "mol"^(-1)...
Text Solution
|
- Which of the following statements about BF(4)^(-) and AIF(6)^(3-) a...
Text Solution
|
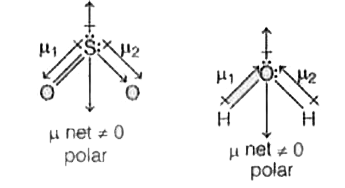
- Statement (A) CO2 has no dipole moment, whereas SO2 and H2O have di...
Text Solution
|
- Assertion (A) Xe atoms in XeF2 are d^2sp^3 hybridised. Reason (R)...
Text Solution
|
- 12 cm^3 of SO2 (g) diffused through a porous membrane in 1 minute. ...
Text Solution
|
- 1 mole of gas A and 1 mole of gas B at 27^@C were pumped into a 24.6 ...
Text Solution
|
- 28 g KOH is required to completely neutralise CO2 produced on heatin...
Text Solution
|
- Which one of following is a disproportionation reaction?
Text Solution
|
- The standard enthalpy of formation of CO (g), CO2 (g) , N2O (g) " and ...
Text Solution
|
- Consider the following reaction in a 1 L closed vessel. N2 + 3H2 if...
Text Solution
|
- If the solubility product of Ni(OH)2 is 4.0 xx 10^(-15) the solubilit...
Text Solution
|
- Identify the reactions in which H2 is liberated ? (i)Zn + NaOH (aq)...
Text Solution
|
- BeH2 can be prepared by the reaction of
Text Solution
|
- AlCl3 in water at pH < 7 forms
Text Solution
|
- Which of the following is knows as silicone ?
Text Solution
|
- Indentify the reactions that occur in photochemical smog . (i) CH2...
Text Solution
|
- IUPAC name of isoprene is
Text Solution
|
- Identify the correct catalyst and reaction conditions for the controll...
Text Solution
|
- The major product (P) formed in the below reaction is H - C -= C - C...
Text Solution
|
- Match the following The correct answer is
Text Solution
|