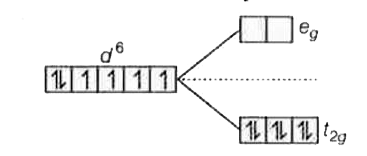
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET ENGINEERING ENTRANCE EXAM QUESTION PAPER (2018)-CHEMISTRY
- The major product (P) formed in the below reaction is H - C -= C - C...
Text Solution
|
- Match the following The correct answer is
Text Solution
|
- How many grams of glucose must be added to 0.5 liter of a solution so ...
Text Solution
|
- Molarity of a 50 mL H2SO4 solution is 10.0 M. If the density of the s...
Text Solution
|
- Limiting molar conductivity of Mg^(2+) and Cl^(-) ions in water is 10...
Text Solution
|
- A particular reaction has a rate constant 1.15 xx 10^(-3) s^(1) . How ...
Text Solution
|
- If the value of 1/n is equal to 1 in Freundlich adsorption isotherm, t...
Text Solution
|
- What is the slag formed during the extraction of iron ?
Text Solution
|
- What is the chemical formula of hypophosphorus acid?
Text Solution
|
- Which of the following ions possesses S - O - S bond ?
Text Solution
|
- Which of the elements possess only one electron in 5d-orbital?
Text Solution
|
- The electronic configuration of Cr in Cr(CO)6 as calculated using cr...
Text Solution
|
- Match the following The correct answer is
Text Solution
|
- Identify the non-reducing sugar from the following
Text Solution
|
- Identify the correct set of functional groups present in aspartame, an...
Text Solution
|
- Which of the following molecules is not chiral ?
Text Solution
|
- The major product obtained in the reaction of bromobenzene with Mg in ...
Text Solution
|
- The major product (Z) of the following chemical reaction is
Text Solution
|
- In the below given synthetic sequence , the product "C" is
Text Solution
|
- What are structure of , X , Y and Z in the above given reaction sequen...
Text Solution
|