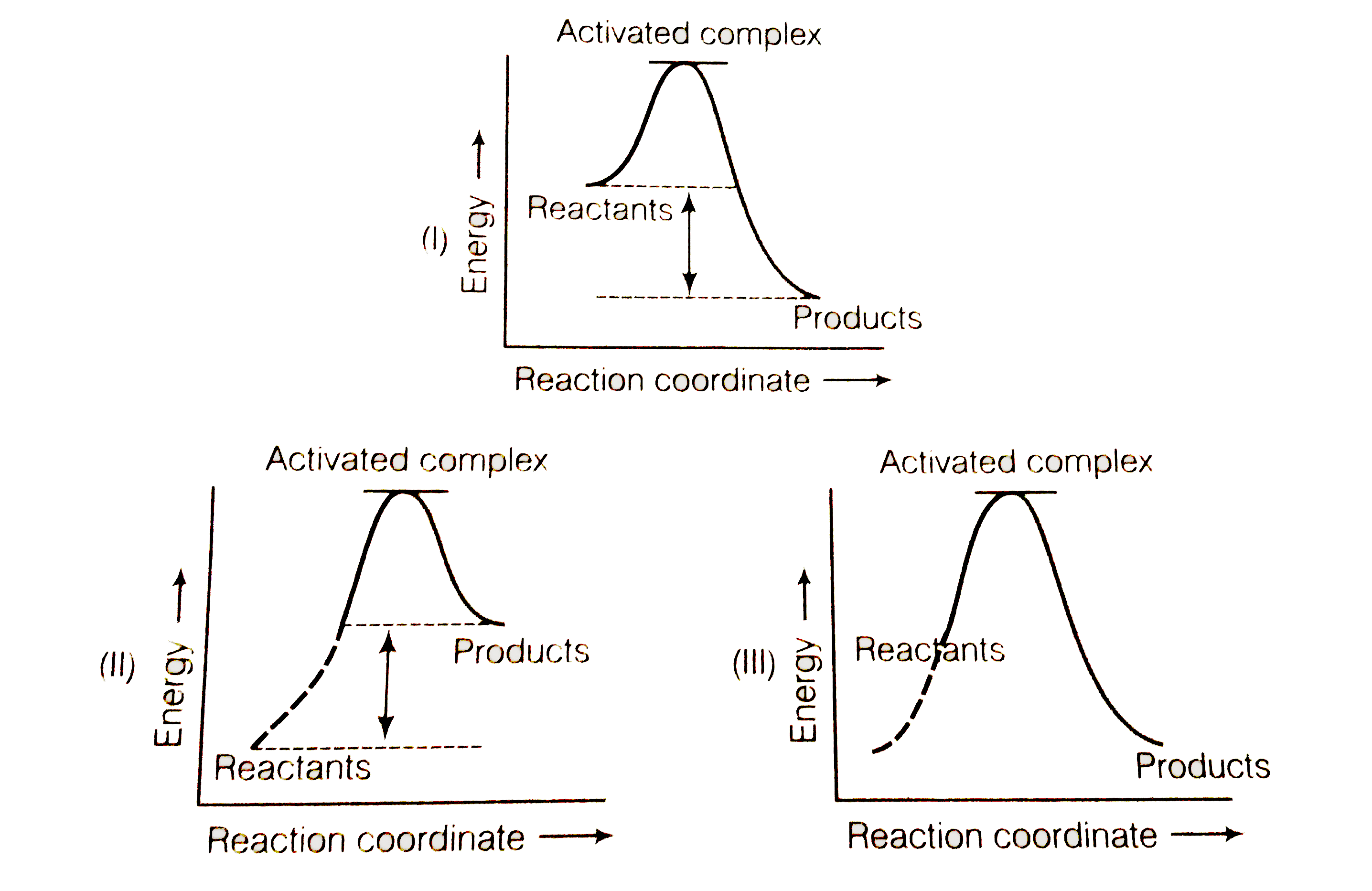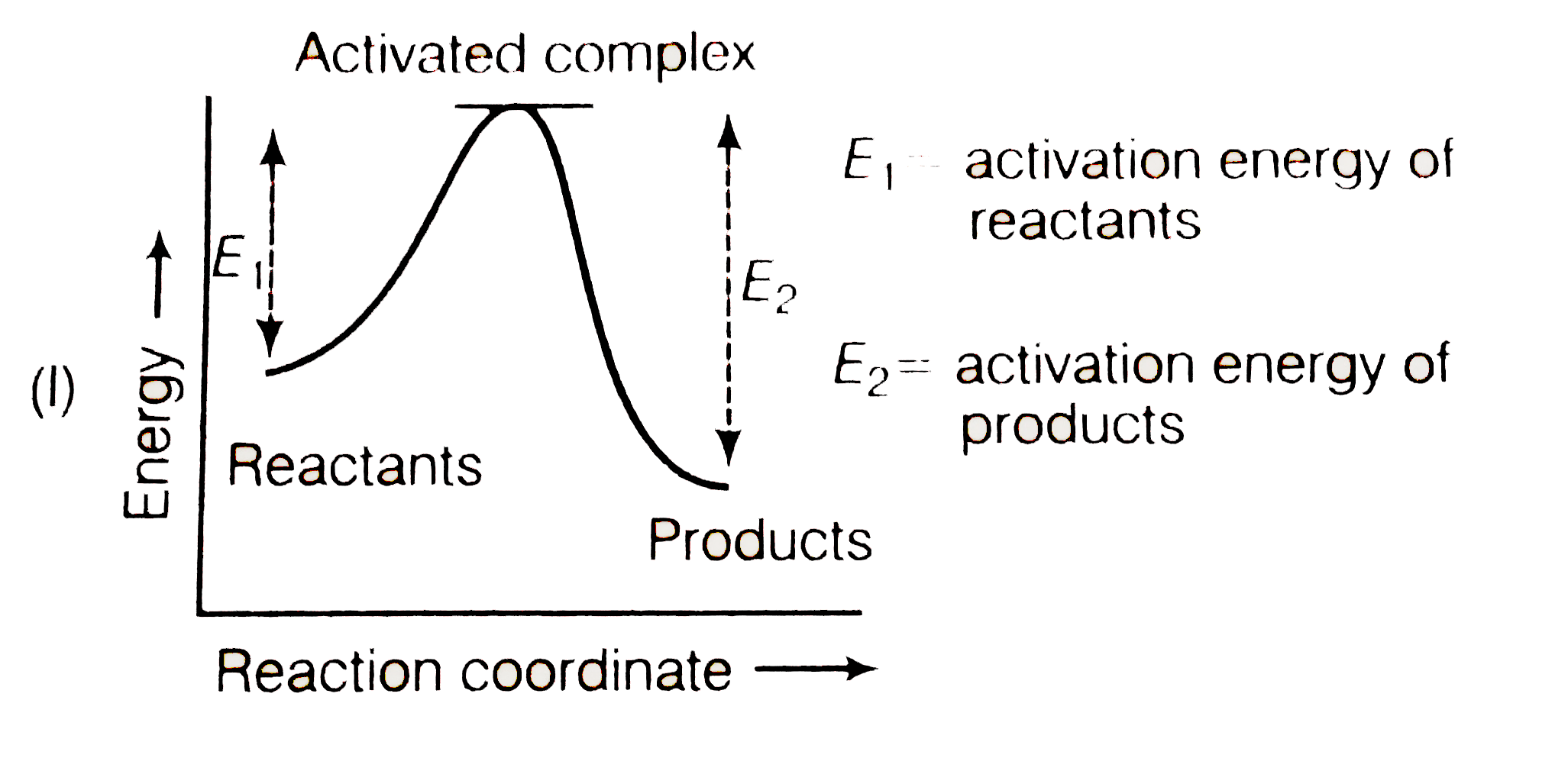A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMICAL KINETICS-Chemical Kinetics
- Which of the following statements is correct ?
Text Solution
|
- Which of the following expression is correct for the rate of reac...
Text Solution
|
- Which of the following graph represents exothermic reaction ?
Text Solution
|
- Rate law for the reaction, A + 2B to C is found to be Rate = k [A] [...
Text Solution
|
- Which of the following statements is incorrect about the collision...
Text Solution
|
- A first order reaction is 50% completed in 1.26 xx 10^(14)s. How much ...
Text Solution
|
- Compounds 'A' and 'B' react according to the following chemical eq...
Text Solution
|
- Which of the following statement is not correct for the catalyst ...
Text Solution
|
- The value of rate constant of a pseudo first order reaction
Text Solution
|
- Consider the reaction A to B. The concentration of both the reactan...
Text Solution
|
- Rate law cannot be determined form balanced chemical equation if â...
Text Solution
|
- Which of the following statements are applicable to a balanced chem...
Text Solution
|
- In any unimoelcular reaction ……. .
Text Solution
|
- For a complex reaction ……… .
Text Solution
|
- At high pressure the following reaction is zero order. " "2N...
Text Solution
|
- During decomposition of an activated complex
Text Solution
|
- According to Maxwell , Boltzmann distriubtion of energy ……… .
Text Solution
|
- In the graph showing Maxwell, Boltzmann distribution of energy â...
Text Solution
|
- Which of the following statements are in accordance with the Arrheeniu...
Text Solution
|
- Mark the incorrent statements.
Text Solution
|