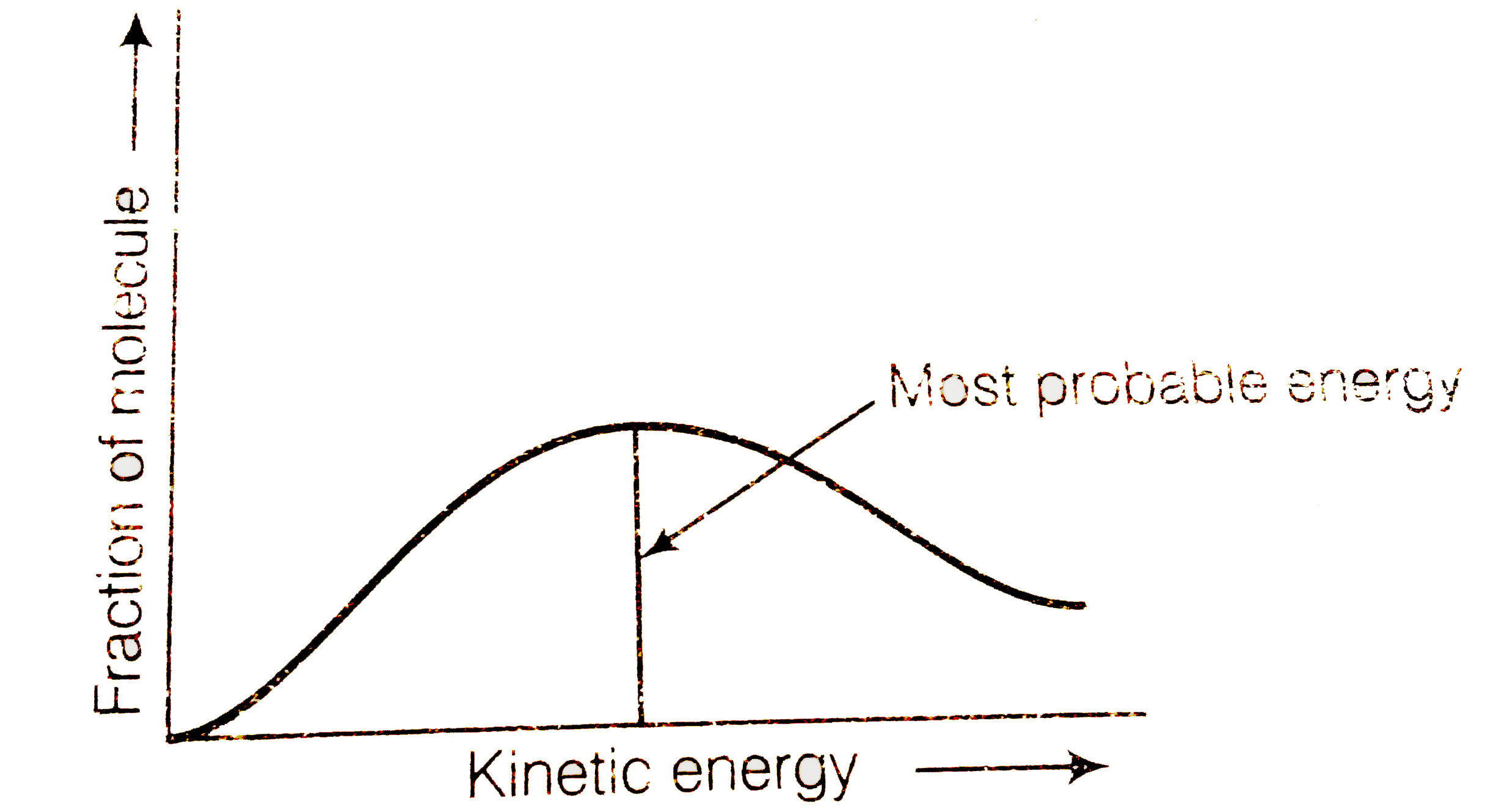
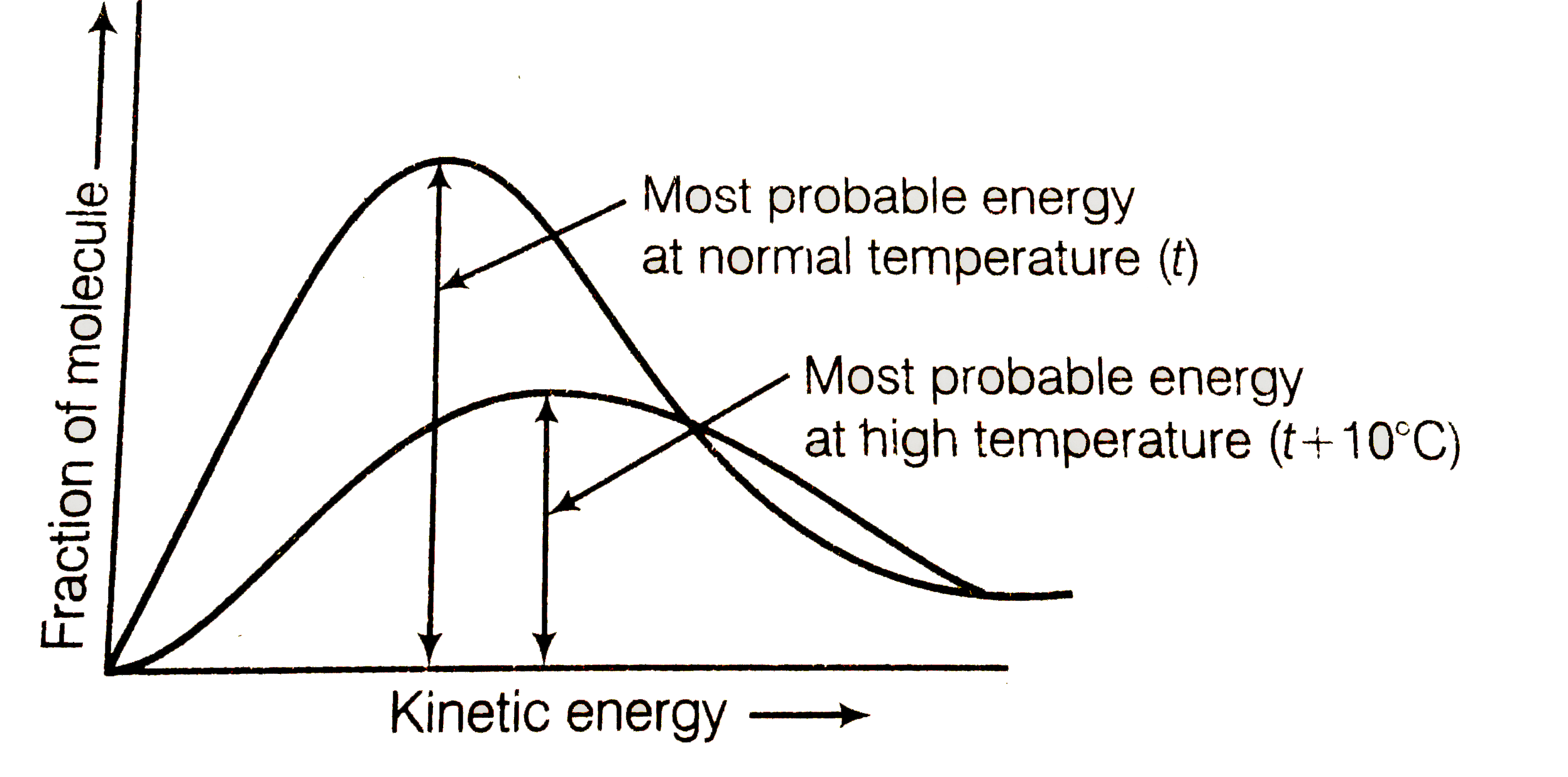
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMICAL KINETICS-Chemical Kinetics
- At high pressure the following reaction is zero order. " "2N...
Text Solution
|
- During decomposition of an activated complex
Text Solution
|
- According to Maxwell , Boltzmann distriubtion of energy ……… .
Text Solution
|
- In the graph showing Maxwell, Boltzmann distribution of energy â...
Text Solution
|
- Which of the following statements are in accordance with the Arrheeniu...
Text Solution
|
- Mark the incorrent statements.
Text Solution
|
- Which of the following graph is correct for a zero order reaction ...
Text Solution
|
- Which of the following graph is correct for a first order reaction ...
Text Solution
|
- State a condition under which a bimolecular reaction is kinetica...
Text Solution
|
- Write the rate equation for the reaction 2A + B to C if the orde...
Text Solution
|
- How can you determine the law of the following reaction? 2NO(g)...
Text Solution
|
- For which type of the reactions, order and molecularity have the sa...
Text Solution
|
- In a reaction if the concentraion of reaction A is tripled, the r...
Text Solution
|
- Derive an expression to caluclate time required time required for co...
Text Solution
|
- For a reaction A+ B to Products, the rate law is -Rate = k[A][B]^(3...
Text Solution
|
- For a certain reacation large fraction of molecules has energy more ...
Text Solution
|
- For a zero order reaction will the molecularity be equal to zero ?...
Text Solution
|
- For a general reaction A to B, plot of concentration of A vs time ...
Text Solution
|
- The reactions between H(2)(g) and O(2)(g) is highly feasuble yet all...
Text Solution
|
- Â Why does the rate of a reaction increase with rise in temperature?
Text Solution
|