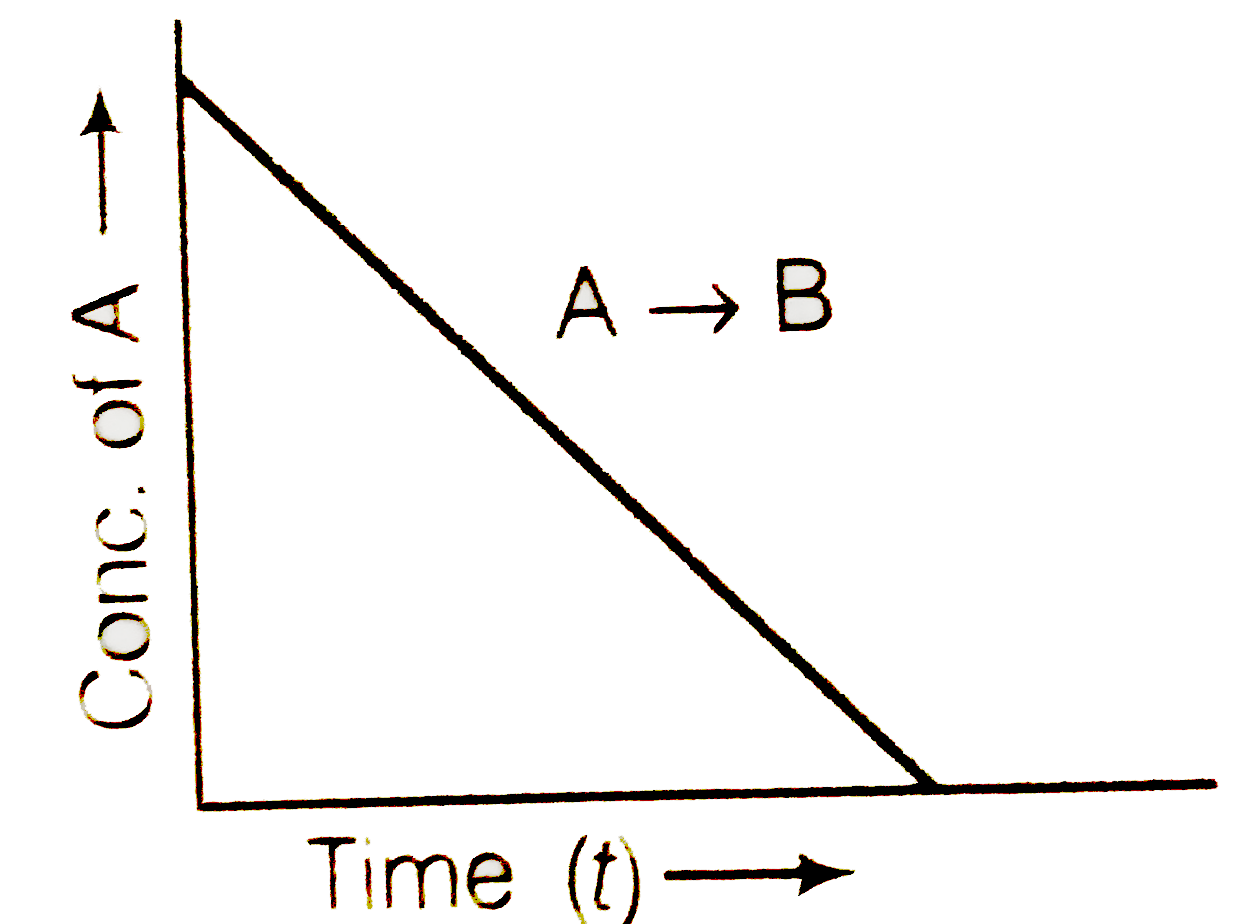Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMICAL KINETICS-Chemical Kinetics
- For a certain reacation large fraction of molecules has energy more ...
Text Solution
|
- For a zero order reaction will the molecularity be equal to zero ?...
Text Solution
|
- For a general reaction A to B, plot of concentration of A vs time ...
Text Solution
|
- The reactions between H(2)(g) and O(2)(g) is highly feasuble yet all...
Text Solution
|
- Â Why does the rate of a reaction increase with rise in temperature?
Text Solution
|
- Â 0xygen is available in plenty in air yet fuels do not burn by themse...
Text Solution
|
- What is the probability of reaction with molecularity higher than thre...
Text Solution
|
- Why does the rate of any reaction generally decreases during the cours...
Text Solution
|
- Thermodynamic feasibility of the reaction alone cannot decide the rate...
Text Solution
|
- Why in the redox titration of kMnO(4) vs oxalic acid, we heat oxali...
Text Solution
|
- Why can't molecularity of any reaction be equal to zero?
Text Solution
|
- Why molecularity is applicable only for elementary reactions and order...
Text Solution
|
- Why can we not determine the order of a rection by taking into ...
Text Solution
|
- Match the grap given in column I with the order of reacting given i...
Text Solution
|
- Match the statements given in Column I abd Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Assertion (A) Order of the reaction can be zero or fractional. Reaso...
Text Solution
|
- Assertion (A) Order and molecularity are same. Reason (R) Order is d...
Text Solution
|
- Assertion (A) The enthalpy of reaction remains constant in the presenc...
Text Solution
|