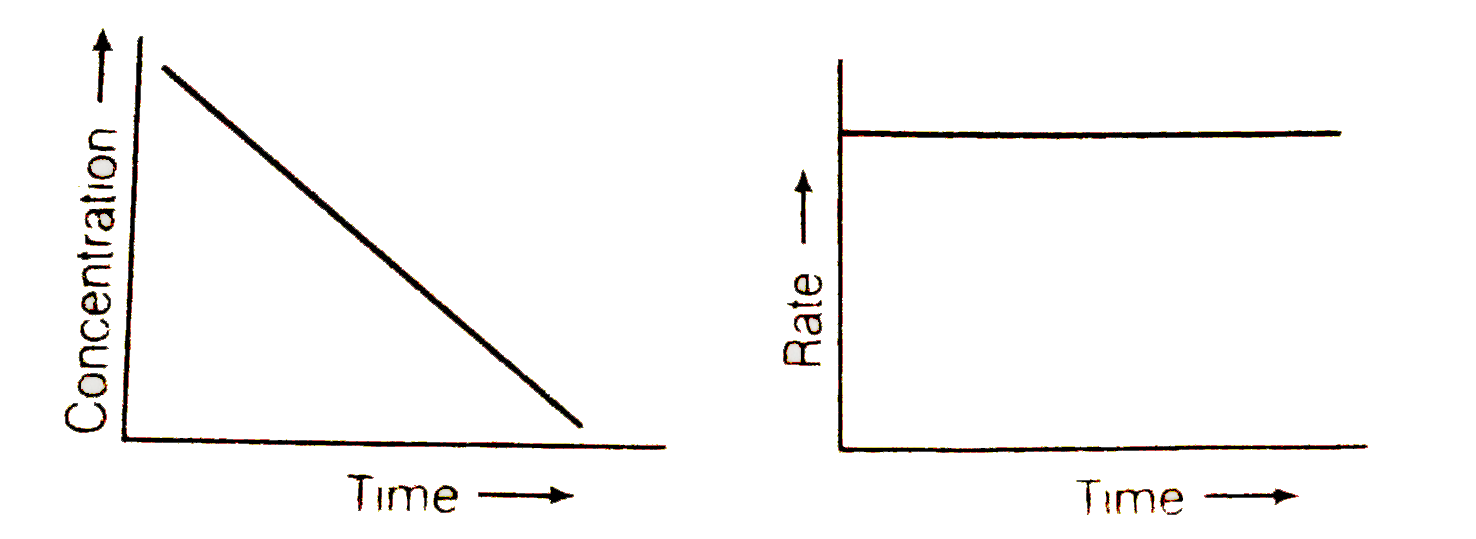
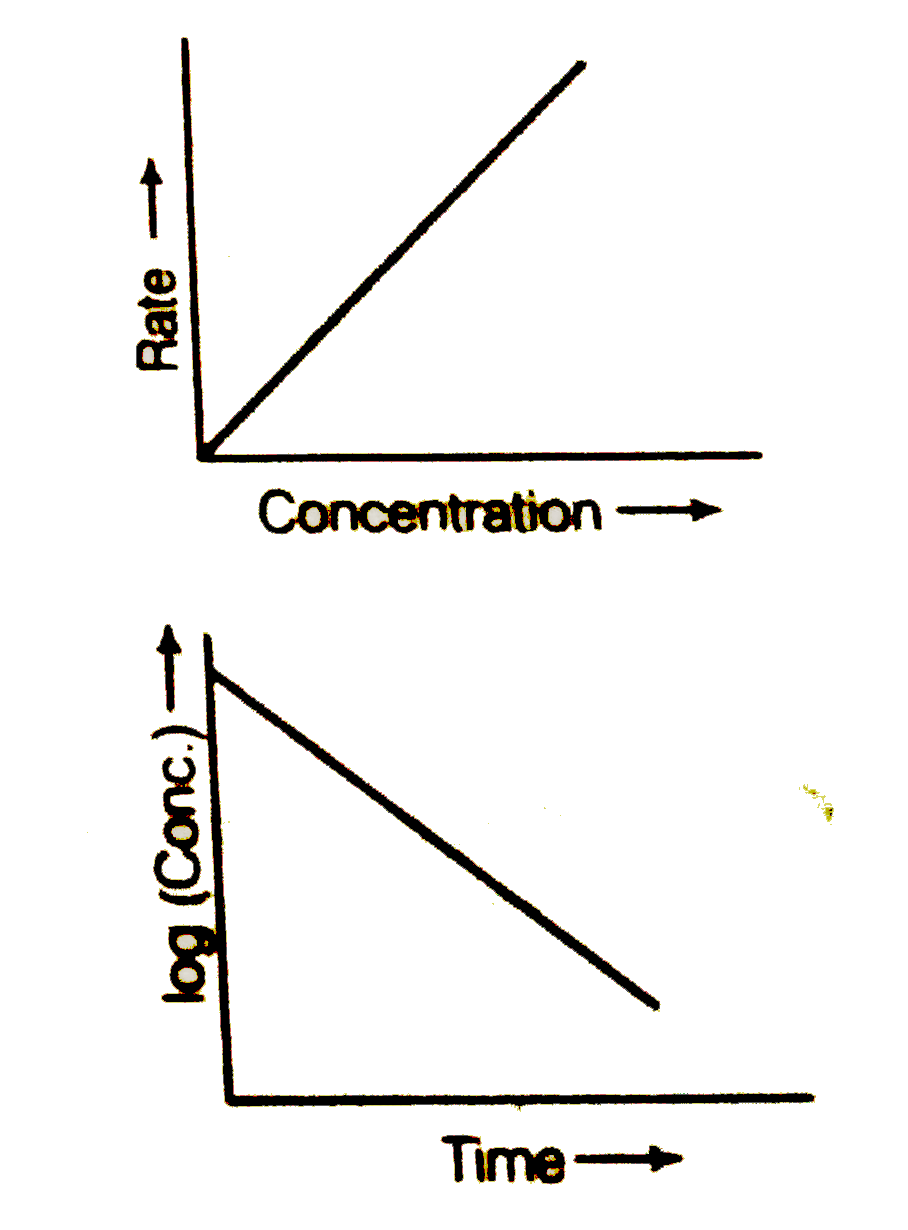
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMICAL KINETICS-Chemical Kinetics
- Why does the rate of any reaction generally decreases during the cours...
Text Solution
|
- Thermodynamic feasibility of the reaction alone cannot decide the rate...
Text Solution
|
- Why in the redox titration of kMnO(4) vs oxalic acid, we heat oxali...
Text Solution
|
- Why can't molecularity of any reaction be equal to zero?
Text Solution
|
- Why molecularity is applicable only for elementary reactions and order...
Text Solution
|
- Why can we not determine the order of a rection by taking into ...
Text Solution
|
- Match the grap given in column I with the order of reacting given i...
Text Solution
|
- Match the statements given in Column I abd Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Match the items of Column I and Column II.
Text Solution
|
- Assertion (A) Order of the reaction can be zero or fractional. Reaso...
Text Solution
|
- Assertion (A) Order and molecularity are same. Reason (R) Order is d...
Text Solution
|
- Assertion (A) The enthalpy of reaction remains constant in the presenc...
Text Solution
|
- Assertion (A) All collision of reactant molecules lead to product form...
Text Solution
|
- Assertion (A) Rate constant determined form Arrhenius equations are...
Text Solution
|
- All energetically effective collisions do not result in a chemical ...
Text Solution
|
- What happes to most probable to the absolute temperatur and the en...
Text Solution
|
- Describe how does the enthalpy of reaction remain unchanged when a cat...
Text Solution
|
- Explain the difference between instantaneous rate of a reaction and ...
Text Solution
|
- With the help of an example explain what is meant by pseudo first or...
Text Solution
|