Recommended Questions
- In the arrangement shown in figure, the piston can smoothly move insid...
Text Solution
|
- Find out bulk stress on the spherical object of radius 10//pi cm Pisto...
Text Solution
|
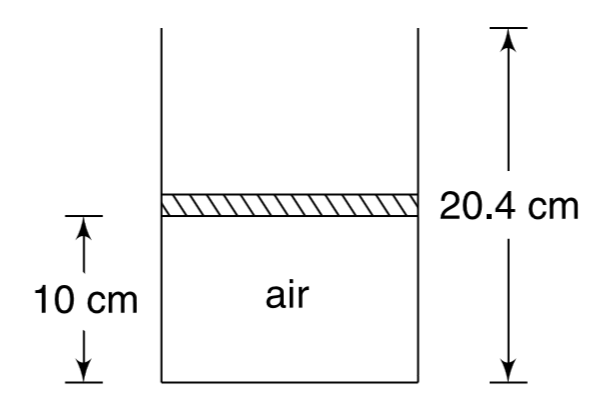
- Air is contained in a piston-cylinder arrangement as shown in Fig. wit...
Text Solution
|
- A tuning fork is used to produce resonance in glass tuve. The length o...
Text Solution
|
- In the arrangement shown in figure, the piston can smoothly move insid...
Text Solution
|
- 2 moles of a diatomic gas are enclosed in a cylinder piston arrangment...
Text Solution
|
- किसी स्वरित्र द्विभुज का उपयोग किसी ऐसी काँच की नलिका में अन्नुकद उत्प...
Text Solution
|
- A vertical cylinder of height 100 cm contains air at a constant temper...
Text Solution
|
- A tuning fork is used to produce resonance in a glass tube . The lengt...
Text Solution
|