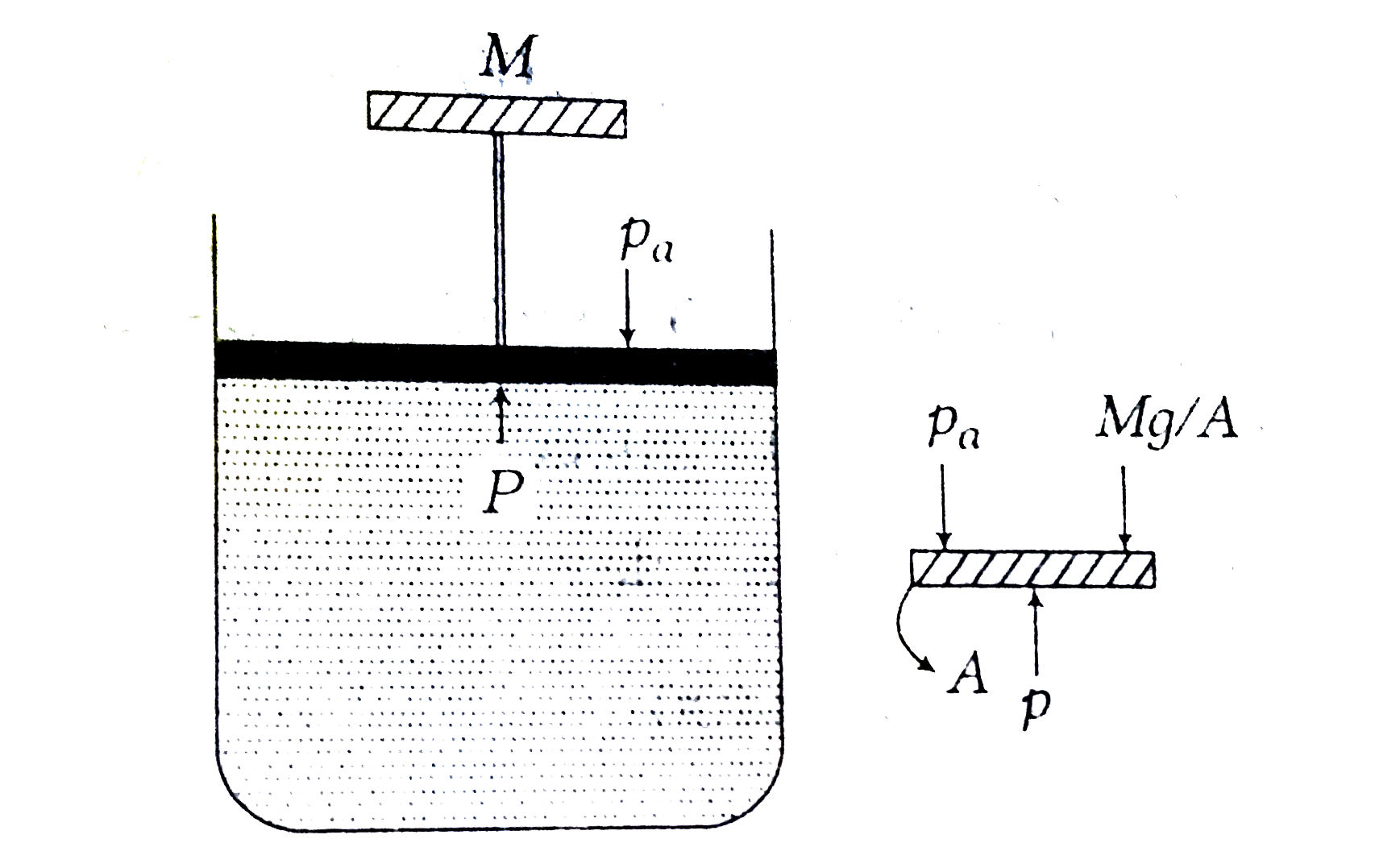A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise B medical entrance special format questions|14 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise Match the columns|5 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise Check point 14.4|25 VideosSUPERPOSITION OF WAVES
DC PANDEY|Exercise Level 2 Subjective|8 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY|Exercise Level 2 Subjective|9 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES-A Tacking it together
- Heat given to a system can be associated with
Text Solution
|
- Boyles's Law is applicable for an
Text Solution
|
- A cylinder containing an ideal gas is in vertical position and has a p...
Text Solution
|
- The absolute zero temperature in Fahrenheit scale is
Text Solution
|
- On which of the following scales of temperature, the temperature is ne...
Text Solution
|
- In a mercury rhermometer the ice point (lower fixed point) is marked a...
Text Solution
|
- On a new scale of temperature (which is linear) and called the W scale...
Text Solution
|
- A centigrade and a Fahrenheit thermometer are dipped in boiling water....
Text Solution
|
- A constant volume gas thermometer show pressure reading of 50cm and 99...
Text Solution
|
- A metal rod of silver at 0^(@)C is heated to 100^(@)C . It's length is...
Text Solution
|
- The volume of a gas at 20^(@)C is 100 cm 3 at normal pressure. If it i...
Text Solution
|
- Coefficient of apparent expansions of mercury is 0.18 xx 10^(-3)//^(0)...
Text Solution
|
- A clock with a metal pendulum beating seconds keeps correct time at 0^...
Text Solution
|
- The graph AB shown in figure is a plot of temperature of a body in deg...
Text Solution
|
- If at same temperature and pressure, the densities for two diatomic ga...
Text Solution
|
- A gas at the temperature 250 K is contained in a closed vessel. If the...
Text Solution
|
- An electron tube was sealed off during manufacture at a pressure of 1....
Text Solution
|
- At what temperature is the root mean square velocity of gaseous hydrog...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- Variation of internal energy with density of 1 "mole" of monatomic gas...
Text Solution
|