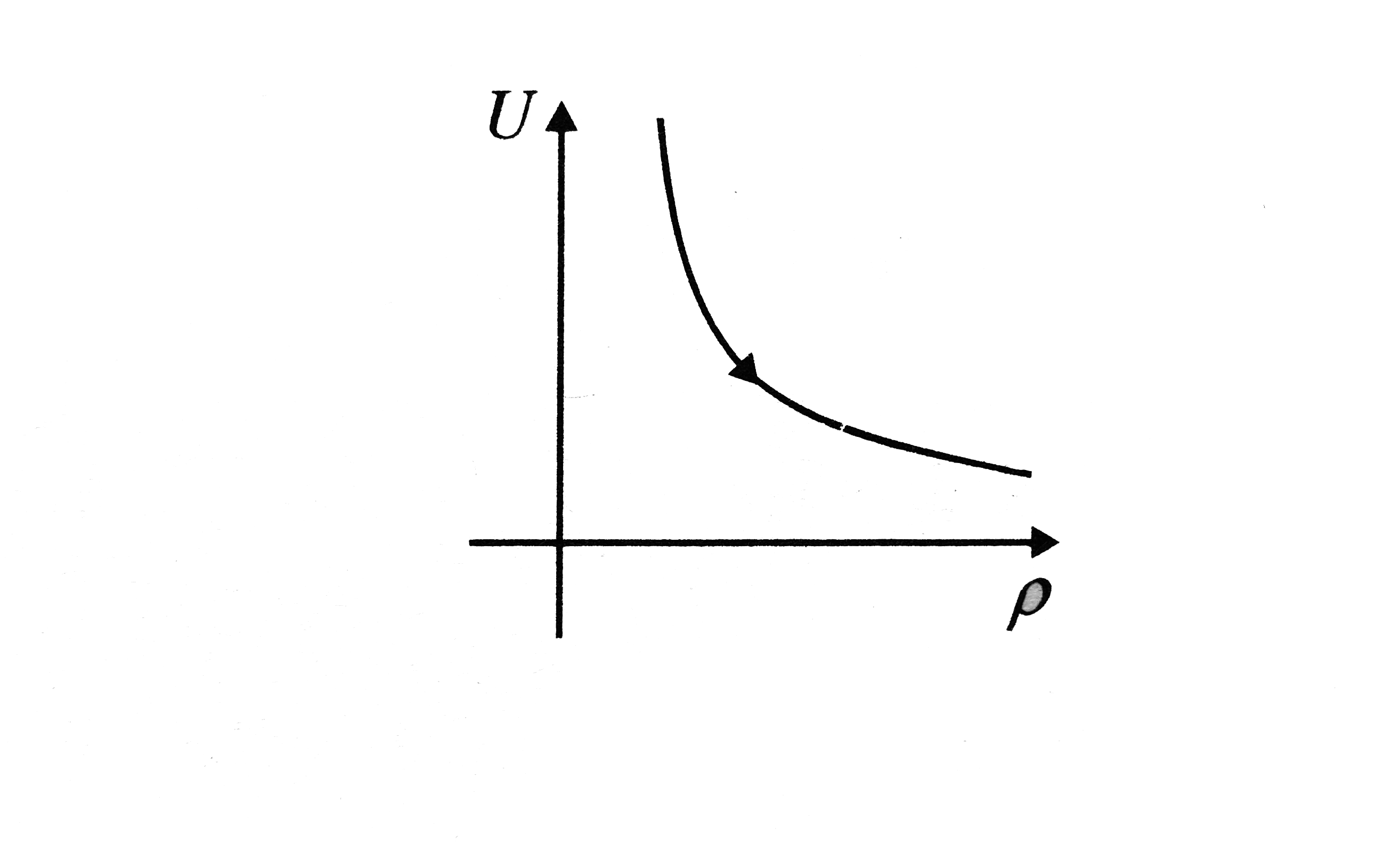
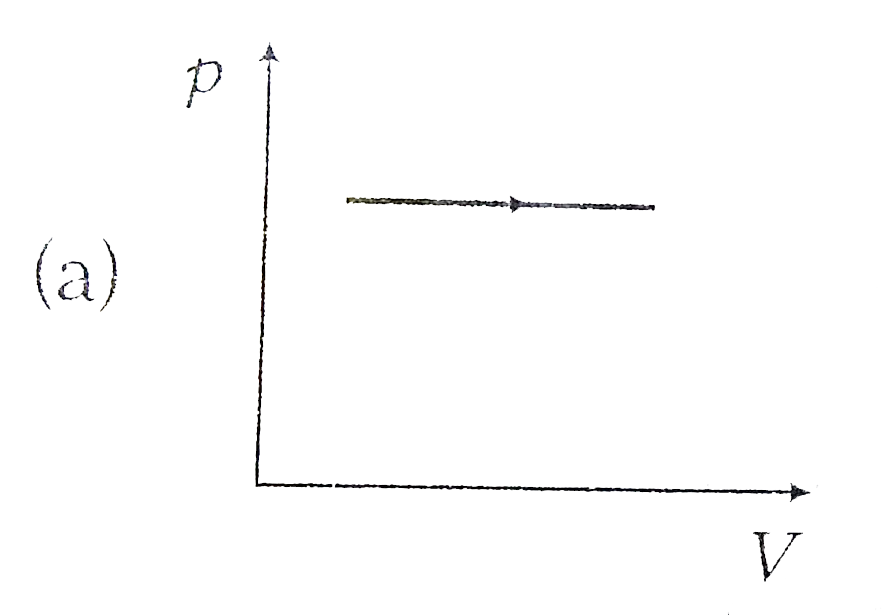
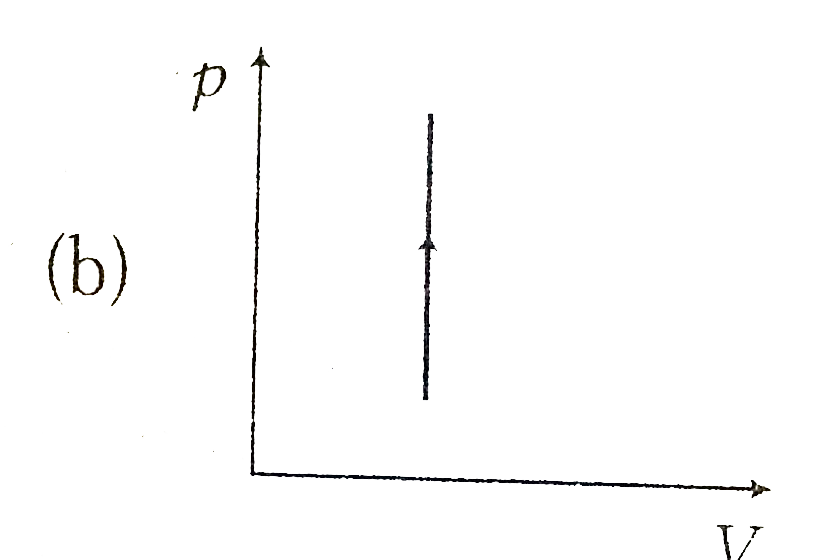
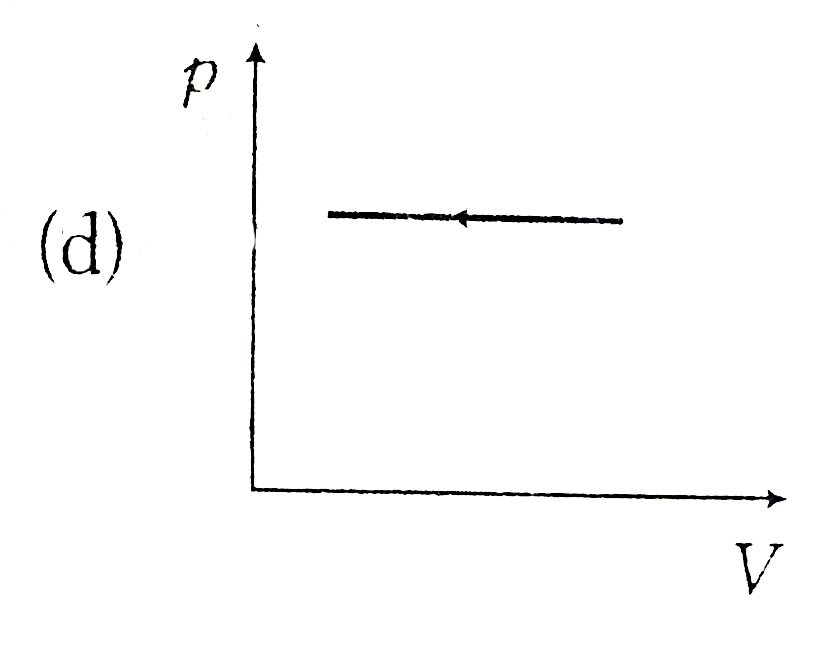
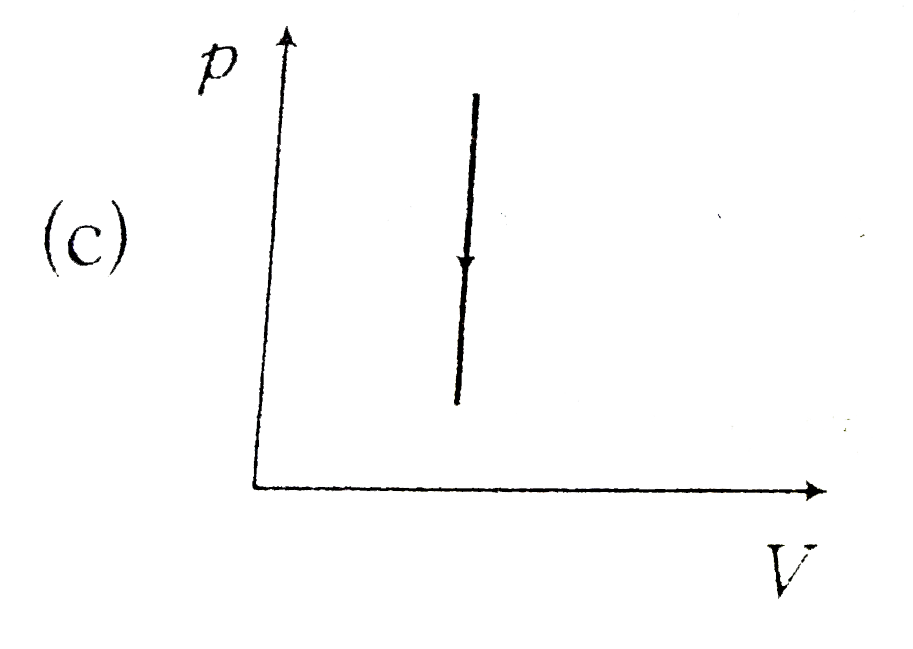A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise B medical entrance special format questions|14 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise Match the columns|5 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise Check point 14.4|25 VideosSUPERPOSITION OF WAVES
DC PANDEY|Exercise Level 2 Subjective|8 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY|Exercise Level 2 Subjective|9 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES-A Tacking it together
- At what temperature is the root mean square velocity of gaseous hydrog...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- Variation of internal energy with density of 1 "mole" of monatomic gas...
Text Solution
|
- Achamber containijng a gas was evacuated till the vacuum attained was ...
Text Solution
|
- A bimetallic strip is made of aluminium and steel (alpha(Al)gtalpha(st...
Text Solution
|
- A uniform metallic rod rotates about its perpendicular bisector with ...
Text Solution
|
- Volume versus temperature graphs for a given mass of an ideal gas are ...
Text Solution
|
- As the temperature is increased, the period of a pendulum
Text Solution
|
- An aluminium sphere is dipped into water. Which of the following is tr...
Text Solution
|
- The radius of a metal sphere at room temperature T is R, and the coeff...
Text Solution
|
- A cubic vessel (with face horizontal + vetical ) contains an ideal gas...
Text Solution
|
- The graph between two temperature scales A and B is shown in Fig. Betw...
Text Solution
|
- 1 mole of H(2) gas is contained in box of volume V= 1.00 m^(3) at T = ...
Text Solution
|
- An inflated rubber balloon contains one mole of an ideal gas has a pre...
Text Solution
|
- A glass vessel of volume V(o) is comopleated filled with volume of the...
Text Solution
|
- The steam point and the ice point of a mercury thermometer are marked ...
Text Solution
|
- Two unifrom brass rod A and B of length l and 2l and radii 2r respect...
Text Solution
|
- The expansion of an ideal gas of mass ma t a constant pressur p is giv...
Text Solution
|
- The root mean square velocity of the molecules in a sample of helium i...
Text Solution
|
- At room temperature, the rms speed of the molecules of a certain diato...
Text Solution
|