Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-ATOMS-MEDICAL ENTRANCES GALLERY
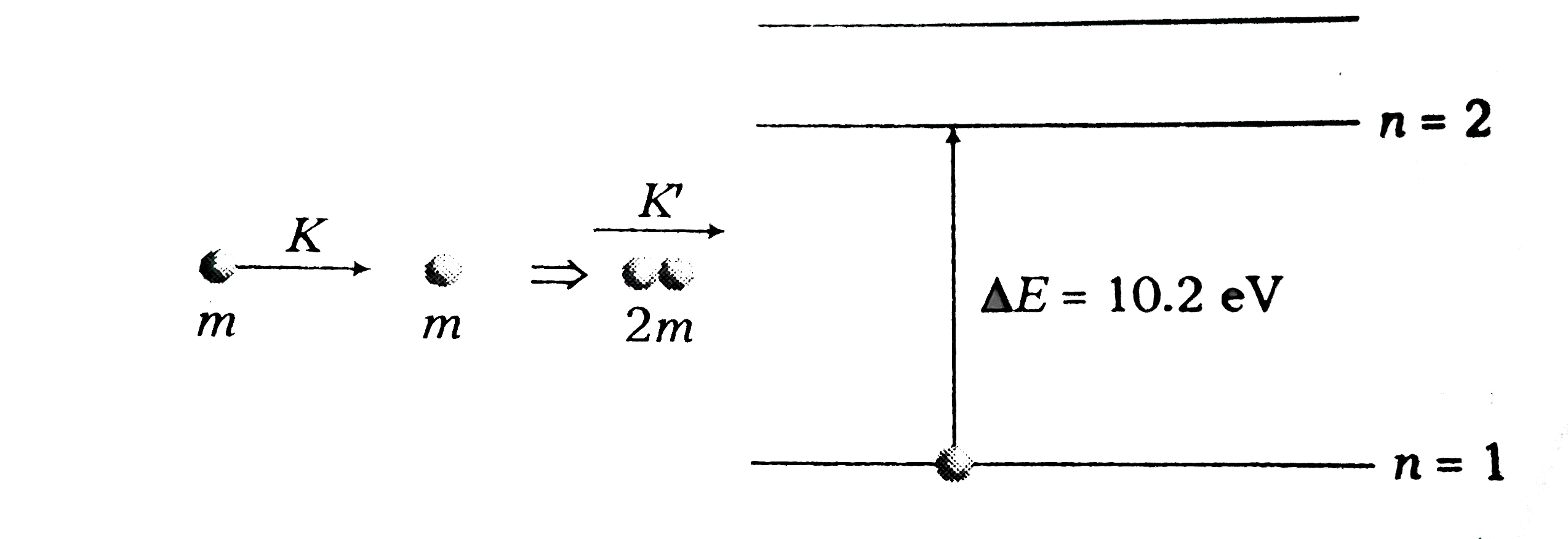
- A moving hydrogen atom makes a head on collision with a stationary hy...
Text Solution
|
- Given the value of Rydberg constant is 10^(7)m^(-1), the waves number ...
Text Solution
|
- When an alpha particle of mass m moving with velocity v bombards on a ...
Text Solution
|
- Electrons with de- Broglie wavelength lambda fall on the target in an ...
Text Solution
|
- If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd ...
Text Solution
|
- Number of spectral lines in hydrogen atom is
Text Solution
|
- Minimum excitation potential of Bohr's first orbit hydrogen atom is
Text Solution
|
- If the frequency of K(alpha), K(beta) and L(alpha) , X-ray lines of a...
Text Solution
|
- The magntic moment (mu) of a revolving electron around the mucleaus v...
Text Solution
|
- The ionization enegry of the electron in the hydrogen atom in its grou...
Text Solution
|
- A photon of wavelength 300nm interacts with a stationary hydrogen atom...
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- What is the wavelength of ligth for the least energetic photon emitted...
Text Solution
|
- The wavelength of k(alpha) X- rays produced by an X - rays tube is 0....
Text Solution
|
- The de-Broglie wavelength of an electron in 4th orbit is (where, r=rad...
Text Solution
|
- The de-Broglie wavelength of an electron is the same as that of a 50 k...
Text Solution
|
- The ionisation energy of hydrogen is 13.6 eV . The energy of the photo...
Text Solution
|
- Hydrogen atom in ground state is excited by a monochromatic radiation ...
Text Solution
|
- If, an electron in hydrogen atom jumps from an orbit of lelvel n=3 to ...
Text Solution
|
- In the spectrum of hydrogen atom, the ratio of the longest wavelength ...
Text Solution
|
- The Rutherford charge experiment proves than an atom consists of
Text Solution
|