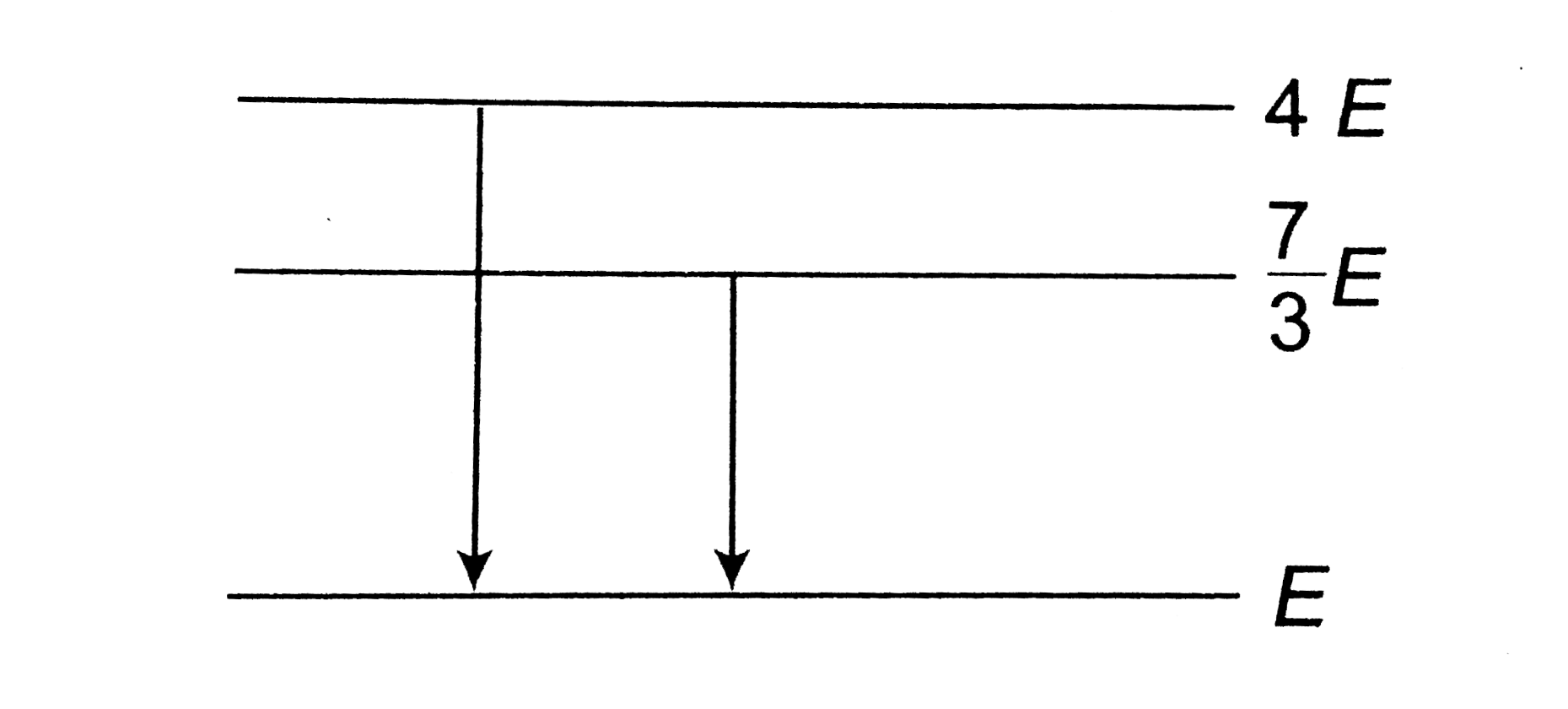
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-ATOMS-Taking it together
- A beam of fast moving alpha particles were directed towards a thin fil...
Text Solution
|
- Figure shows the enegry levels P, Q, R, S and G of an atom where G is ...
Text Solution
|
- The binding energy of a H-atom considering an electron moving aroun...
Text Solution
|
- If the intensity of an X-ray becomes (I(0))/(2) from I(0) after travel...
Text Solution
|
- In the following figure the energy levels of hydrogen atom have been s...
Text Solution
|
- The ionization energy of the electron in the hydrogen atom in its grou...
Text Solution
|
- If an electron is revolving around the hydrogen nucleus at a distance ...
Text Solution
|
- Imagine an atom made of a proton and a hypothetical particle of double...
Text Solution
|
- For a certain atom, there are energy levels A,B,C corresponds to energ...
Text Solution
|
- The electron in a hydrogen atom makes a transition n(1) rarr n(2), whe...
Text Solution
|
- If elements with principal quantum number ngt 4 were not allowed in na...
Text Solution
|
- The following diagram indicates the energy levels of a certain atom wh...
Text Solution
|
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- When the voltage applied to and X-ray tube is increases from V(1)=10KV...
Text Solution
|
- The ionisation potential of hydrogen atom is -13.6 eV. An electron in...
Text Solution
|
- The distance of closest approach of an alpha-particle fired towards a ...
Text Solution
|
- The first excited state of hydrogen atom is 10.2 eV above its ground s...
Text Solution
|
- Hydrogen (1H^(1)) Deuterium (1H^(2)) singly ionized helium (2He^())^...
Text Solution
|
- Suppose an electron is attracted toward the origin by a force(k)/(r ) ...
Text Solution
|
- A hydrogen like atom with atomic number Z is in an excited state of q...
Text Solution
|