Similar Questions
Explore conceptually related problems
Recommended Questions
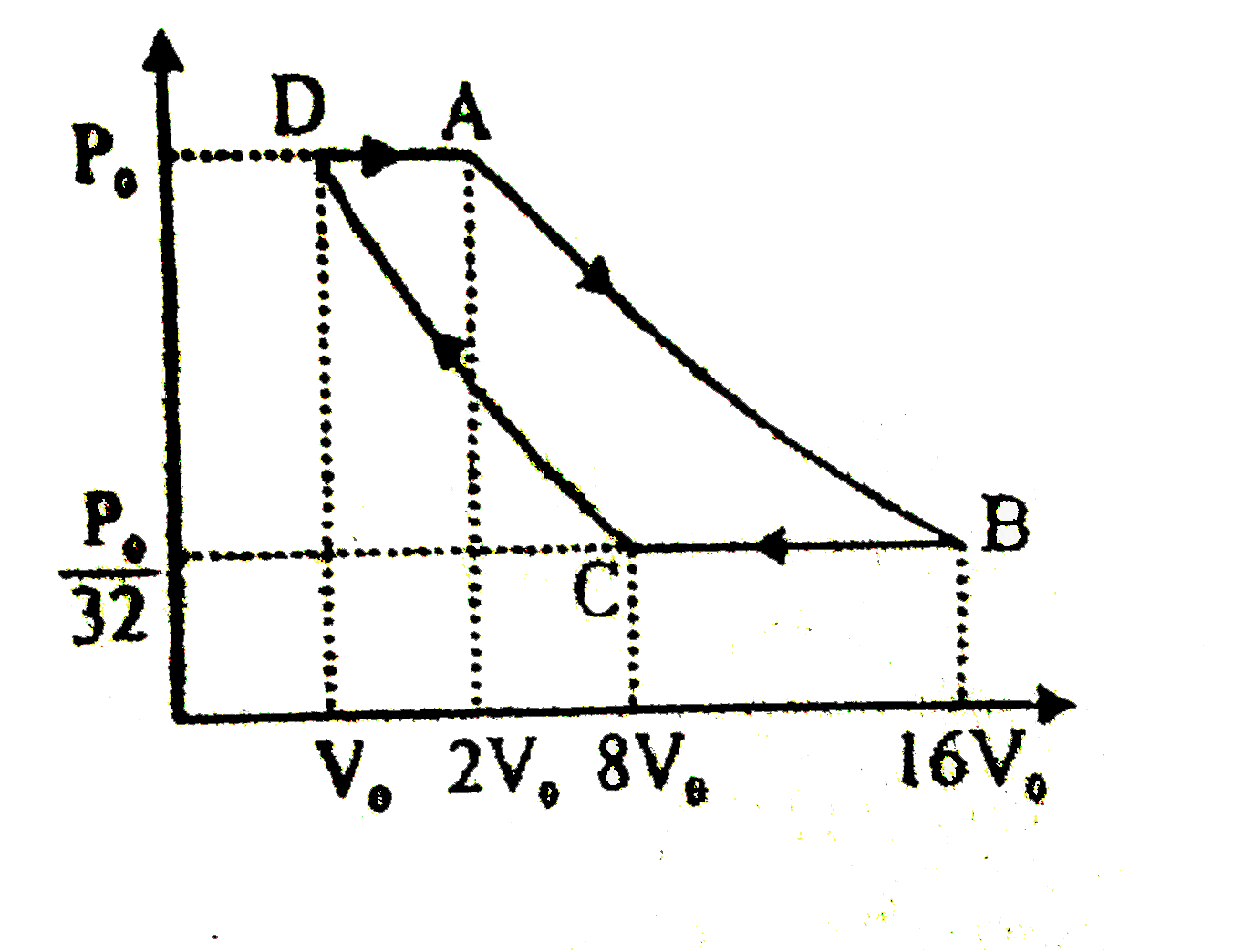
- Two moles of an ideal gas goes under the process shown in figure. AB a...
Text Solution
|
- The process on an ideal gas, shown in figure,is
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- On a T-P diagram, two moles of ideal gas perform process AB and CD. If...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- A gas is allowed to expand reversibly under adiabatic conditions. What...
Text Solution
|
- Two moles of an ideal gas goes under the process shown in figure. AB a...
Text Solution
|
- Two moles of an ideal gas goes under the process shown in figure. AB a...
Text Solution
|