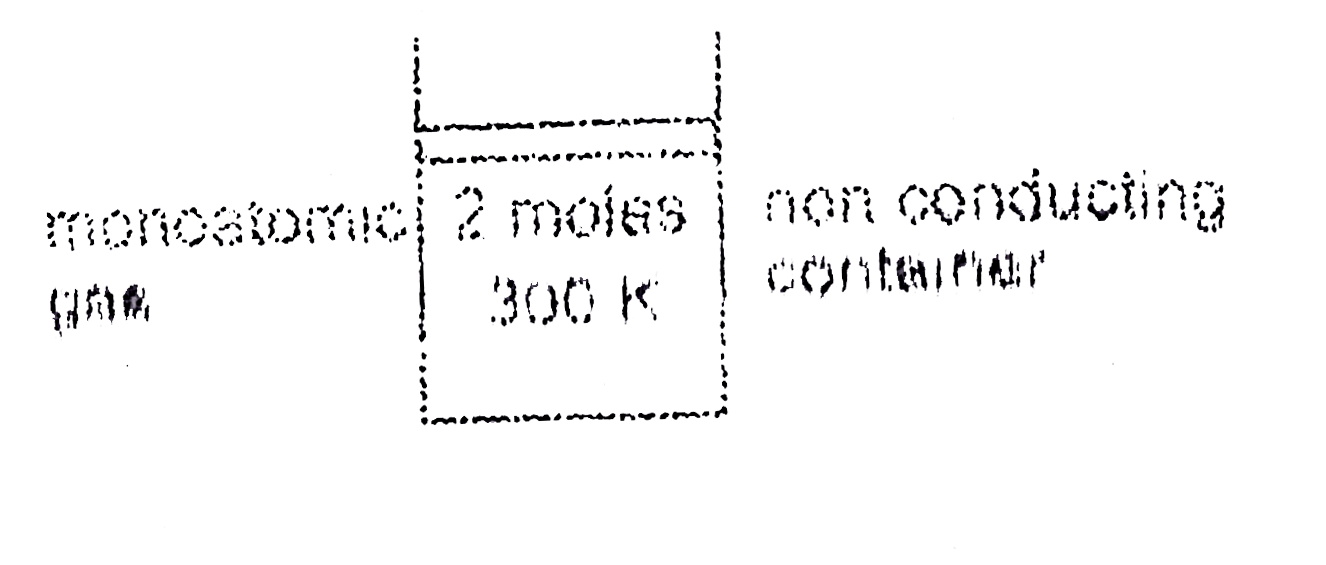Similar Questions
Explore conceptually related problems
Recommended Questions
- Two moles of a monoatomic gas at 300K are kept in a non conducting con...
Text Solution
|
- Temperature of four moles of a monoatomic gas is increased by 300K in ...
Text Solution
|
- Temperature of two moles of an ideal gas is increased by 300K in a pro...
Text Solution
|
- Two moles of a diatomic gas at 300 K are kept in a nonconducting conta...
Text Solution
|
- Three moles of an ideal gas kept at a constant temperature of 300K are...
Text Solution
|
- Some gas at 300K is enclosed in a container. Now the container is plac...
Text Solution
|
- Temperature of 1 mole of an ideal gas is increased from 300K to 310K u...
Text Solution
|
- Two moles of a monoatomic gas at 300K are kept in a non conducting con...
Text Solution
|
- Comprehension-2 A mono atomic ideal gas is filled in a non conducting...
Text Solution
|