Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-THERMODYNAMICS-NUMERICALS WITH SOLUTIONS
- A steel tape 1 m long is correctly cailbrated for temperature of 27.0^...
Text Solution
|
- A hole is drilled in a copper sheet. The diameter of the hole is 4.24c...
Text Solution
|
- A brass wire 1.8 m long at 27^(@)C is held taut with a little tension ...
Text Solution
|
- A 10 kW drilling machine is used to drill a bore in a small aluminium ...
Text Solution
|
- A copper block of mass 2.5 kg is heated in a furance to temperature of...
Text Solution
|
- A geyser heats water flowing at the rate of 3.0 litres per minute from...
Text Solution
|
- What amount of heat must be supplied to 2.0xx10^(-2)kg of N(2) (at roo...
Text Solution
|
- A tyre pumped at a pressure of 3.375 atm and at 27^(@)C suddenly burst...
Text Solution
|
- The volume of 1 kg water is reduced by 91cm^(3) on melting. Calculate ...
Text Solution
|
- A gas expands from 75 litre to 125 litre at a constant pressure of 4 a...
Text Solution
|
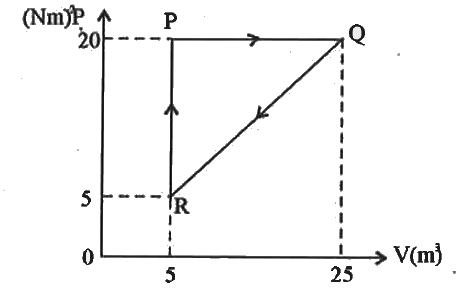
- Calculate the amount of work done in taking the gas from the state (i)...
Text Solution
|
- 1g of water at atmospheric pressure and at 100^(@)C, 1671 cm^(3) of w...
Text Solution
|
- The efficiency of a Carbot's heat engine is 0.25. If on reducing the t...
Text Solution
|
- The volume of N(2) gas increases from 1 cm^(3) "to" 100 cm^(3) at ...
Text Solution
|
- The ratio of specific heats of a gas in 1.40. There are 200 moles of g...
Text Solution
|
- The working substance in a Carnot's heat engine absorbs 5xx10^(6)J of...
Text Solution
|
- Caclulate the change in entropy of a 1000 kg of water converted into s...
Text Solution
|
- Under an increase of pressure of atmosphere, the volume of 1m^(3) of i...
Text Solution
|
- A refrigerator is driven by a 1HP motor having an efficiency of 80%. T...
Text Solution
|
- A Carnot heat engine absorbs 600J of heat from source of temperature 8...
Text Solution
|