Text Solution
Verified by Experts
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
SUBHASH PUBLICATION|Exercise NUMERICALS WITH SOLUTIONS|21 VideosDUAL NATURE OF RADIATION AND MATTER
SUBHASH PUBLICATION|Exercise THREE MARK QUESTIONS WITH ANSWERS|5 VideosCURRENT ELECTRICITY
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|29 VideosELECTRIC CHARGES AND FIELDS
SUBHASH PUBLICATION|Exercise NUMERICALS WITH SOLUTIONS|21 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-DUAL NATURE OF RADIATION AND MATTER-FIVE MARK QUESTIONS WITH ANSWERS
- Write any three experimental observations of photoelectric effect
Text Solution
|
- Give Einstein's explanation of photoelectric effect.
Text Solution
|
- What are matter waves? Derive an expression for the de Broglie wave le...
Text Solution
|
- Draw a neat labelled diagram of experimental arrangement of Davisson -...
Text Solution
|
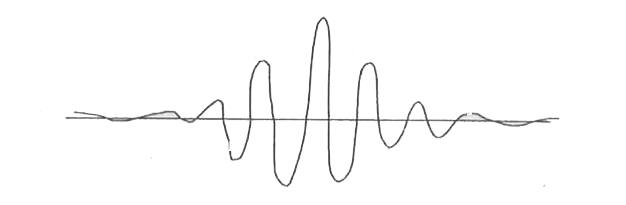
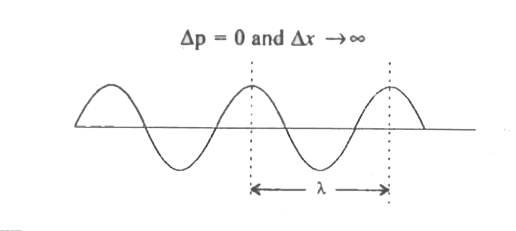
- Explain Werner Heisenberg's uncertainty principle (qualitative).
Text Solution
|