Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Solved Problem|1 VideosMETALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Exercise|305 VideosCHEMICAL REACTIONS AND EQUATIONS
LAKHMIR SINGH & MANJIT KAUR|Exercise QUESTIONS BASED ON HIGH ORDER THINKING SKILLS (HOTS)|1 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise VERY SHORT ANSWER TYPE|6 Videos
Similar Questions
Explore conceptually related problems
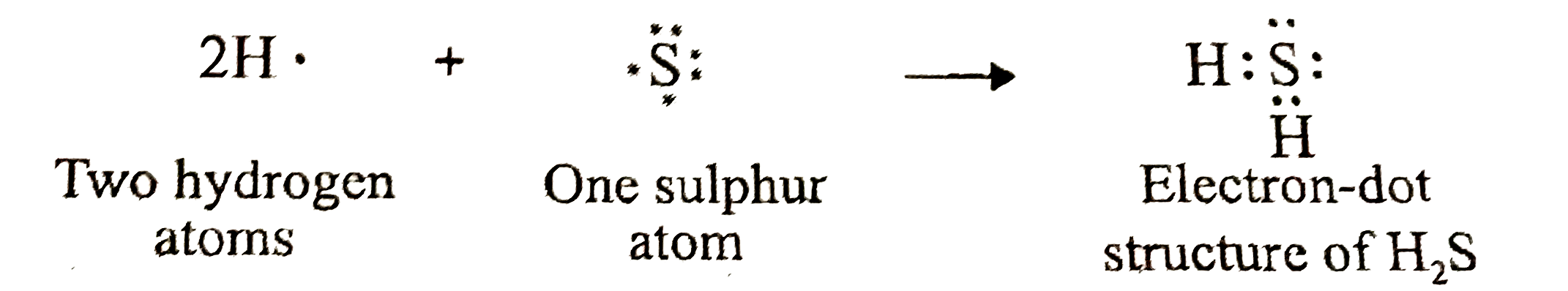
 .
.