Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
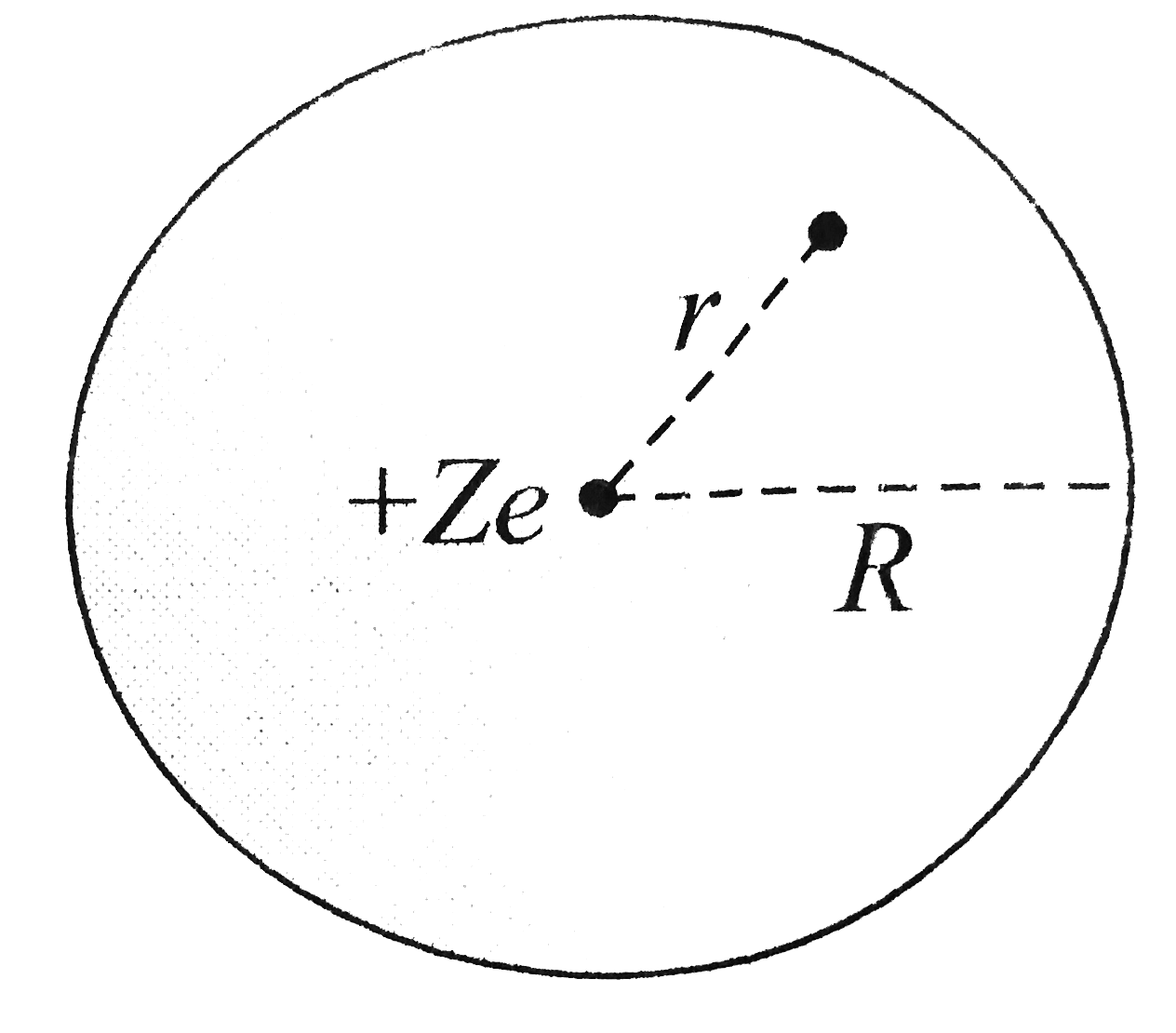
- According to early model of an atom,the atom is considered it to have ...
Text Solution
|
- According to early model of an atom, the atom is considered it to have...
Text Solution
|
- An early model for an atom considered it to have a positively charged ...
Text Solution
|
- प्रारम्भ में परमाणु के मॉडल (atomic model) के लिए यह माना गया था कि प्...
Text Solution
|
- According to early model of an atom,the atom is considered it to have ...
Text Solution
|
- The classical concept of atomic structure is that negative charg...
Text Solution
|
- परमाणु के प्रारंभिक प्रतिरूप में यह माना गया था कि आवेश Ze का बिंदु आम...
Text Solution
|
- An early model for an atom considered to have a positively charged poi...
Text Solution
|
- An early model for an atom consider it to have a positively charged p...
Text Solution
|