A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)|15 VideosCHEMICAL KINETICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION B : Objective Type Questions)|20 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE|Exercise Assignment Section J (Aakash Challengers Questions)|10 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH INSTITUTE|Exercise Assignment ( SECTION - A)|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-CHEMICAL KINETICS-ASSIGNMENT (SECTION C : Previous Year Questions)
- For the reaction A + B products, it is observed that: (1) on doublin...
Text Solution
|
- The rate constant k(1) and k(2) for two different reactions are 10^(16...
Text Solution
|
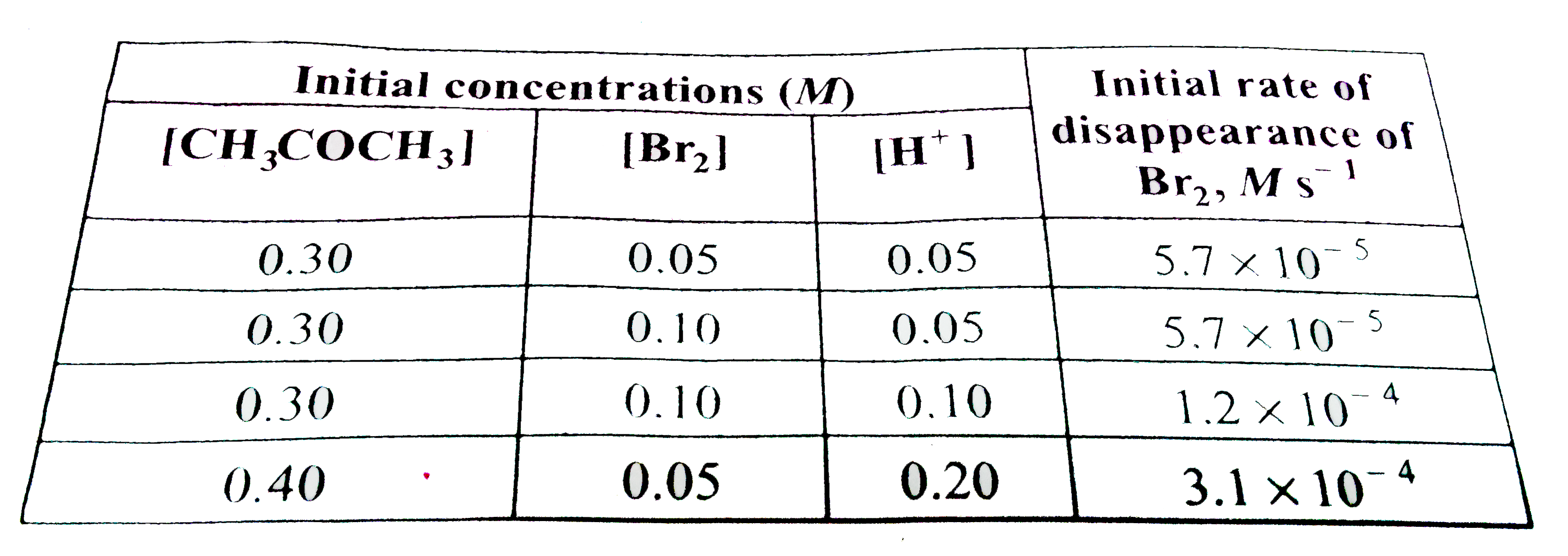
- The bromination of acetone that occurs in acid solution is represente...
Text Solution
|
- In a first order reaction, A rarrB, if K is rate constant and initial ...
Text Solution
|
- The reaction obey I order with respect to H(2) and ICl both. H(2) (g...
Text Solution
|
- If 60% of a first order reaction was completed in 60 minutes, 50% of t...
Text Solution
|
- for the reaction, 2A + B rarr 3C + D, which of the following does not ...
Text Solution
|
- For this reaction N(2(g))+3H(2(g))to2NH(3(g)) The relation be...
Text Solution
|
- For a first order reaction A rarrB , the reaction rate at reactant con...
Text Solution
|
- A nuclide of an alkaine earth metal undergoes radioactive deacy by em...
Text Solution
|
- The rate of reaction between two A and B decreases by factor 4 if the ...
Text Solution
|
- A reaction is 50 % complete in 2 hours and 75 % complete in 4 hours th...
Text Solution
|
- Activation energy (Ea) and rate constants (k1 and k2) of a chemical re...
Text Solution
|
- The half life of 2g sample of radioactive nuclide 'X' is 15 min. The h...
Text Solution
|
- The rate of reaction. 2N(2)O(5) to 4NO(2) + O(2) can be written in...
Text Solution
|
- For the following reaction C6H(12)(aq)+H2(g)hArrC6H(14)O6(aq) Which...
Text Solution
|
- A chemical reaction proceeds into the following steps Step I, 2A hAr...
Text Solution
|
- The data for the reaction: A + B overset(k)rarr C. |{:("Experiment",...
Text Solution
|
- Half - life for radioactive .^(14)C is 5760 years. In how many years 2...
Text Solution
|
- A chemical reaction has catalyst X. Hence X
Text Solution
|