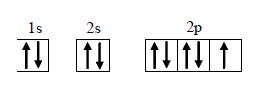Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC PROPERTIES OF ELEMENTS
VIDYAMANDIR CLASSES (VMC MODULES)|Exercise Practice Exercise|30 VideosPERIODIC PROPERTIES OF ELEMENTS
VIDYAMANDIR CLASSES (VMC MODULES)|Exercise In Chapter Exercise-A|11 VideosPERIODIC PROPERTIES OF ELEMENTS
VIDYAMANDIR CLASSES (VMC MODULES)|Exercise In Chapter Exercise-F|7 VideosQUIZ
VIDYAMANDIR CLASSES (VMC MODULES)|Exercise Chemistry|15 Videos
Similar Questions
Explore conceptually related problems
VIDYAMANDIR CLASSES (VMC MODULES)-PERIODIC PROPERTIES OF ELEMENTS -Solved Examples
- The ground state electron configurations listed here are incorrect. Id...
Text Solution
|
- The ground state electron configurations listed here are incorrect. Id...
Text Solution
|
- Draw orbital diagrams for atoms with the following electronic configur...
Text Solution
|
- Draw orbital diagrams for atoms with the following electronic configur...
Text Solution
|
- Draw orbital diagrams for atoms with the following electronic configur...
Text Solution
|
- The first four ionization energies of an element are 738 kJ/mole, 1450...
Text Solution
|
- For Na^(+),Mg^(2+),F^(-) and O^(2-), the correct order of increasing i...
Text Solution
|
- Arrange the following isoelectronic species (O^(2-) , F^(-) , Na^+, Mg...
Text Solution
|
- Which oxide is more basic, MgO or BaO? Whay?
Text Solution
|
- Write balanced equations for the reactions between each of the followi...
Text Solution
|
- Write balanced equations for the reactions between each of the followi...
Text Solution
|
- Write balanced equations for the reactions between each of the followi...
Text Solution
|
- Arrange the elements in each of the following groups in increasing ord...
Text Solution
|
- Arrange the elements in each of the following groups in increasing ord...
Text Solution
|
- Two atoms have the electron configurations 1s^2 2s^2 2p^6 and 1s^2 2s^...
Text Solution
|
- Why are the electron affinities of alkaline earth metals nearly zero?
Text Solution
|
- Account for the decrease in first ionization energy between phosphorou...
Text Solution
|
- Among the elements of the third period (Na to Ar), pick out the elemen...
Text Solution
|
- Among the elements of the third period (Na to Ar), pick out the elemen...
Text Solution
|
- Among the elements of the third period (Na to Ar), pick out the elemen...
Text Solution
|