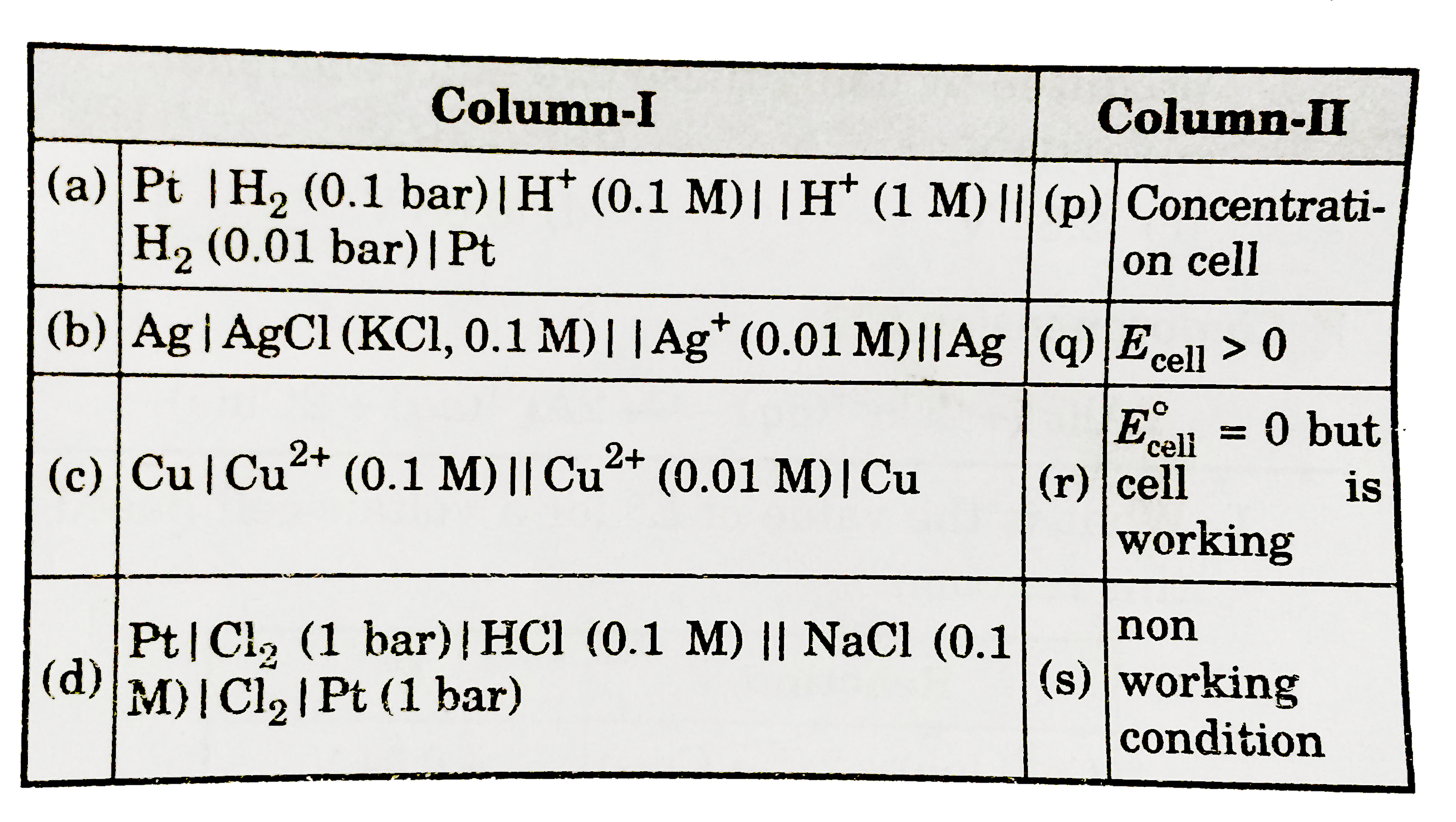
Similar Questions
Explore conceptually related problems
Recommended Questions
- Match matrix (E(Ag^(+)//Ag)^(@)=0.8K(sp)(AgCl)=10^(-10))
Text Solution
|
- Consider the electrode Ag|AgCl(s),Cl^(c-)(0.1M),i.e., silver electrode...
Text Solution
|
- K(sp) of AgCl in water at 25^(@)C is 1.8xx10^(-10). If 10^(-5) mole of...
Text Solution
|
- Match matrix (E(Ag^(+)//Ag)^(@)=0.8K(sp)(AgCl)=10^(-10))
Text Solution
|
- The K(sp) for AgCl at 298 K is 1.0xx10^(-10) . Calculate E for Ag^(+)/...
Text Solution
|
- The standard reduction potential of Ag^(+)|Ag electrode is 0.80 volt. ...
Text Solution
|
- Cu(s)|Cu^(+2)(aq,10^(-3))||Ag^(+)(10^(-5)M)|Ag(s) if E(Cu^(+2)//Cu=0...
Text Solution
|
- Find the K(sp) of AgCl from the following data. The standard electrode...
Text Solution
|
- The K(sp) for AgCl at 298 K is 1.0xx10^(-10) . Calculate the electrode...
Text Solution
|