A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-GEOMETRICAL OPTICS-EXERCISE -01
- If the intermolecules forces vanish away, the volume occupied by the m...
Text Solution
|
- A refrigerator converts 100 g of water at 25^(@)C into ice at -10^(@)C...
Text Solution
|
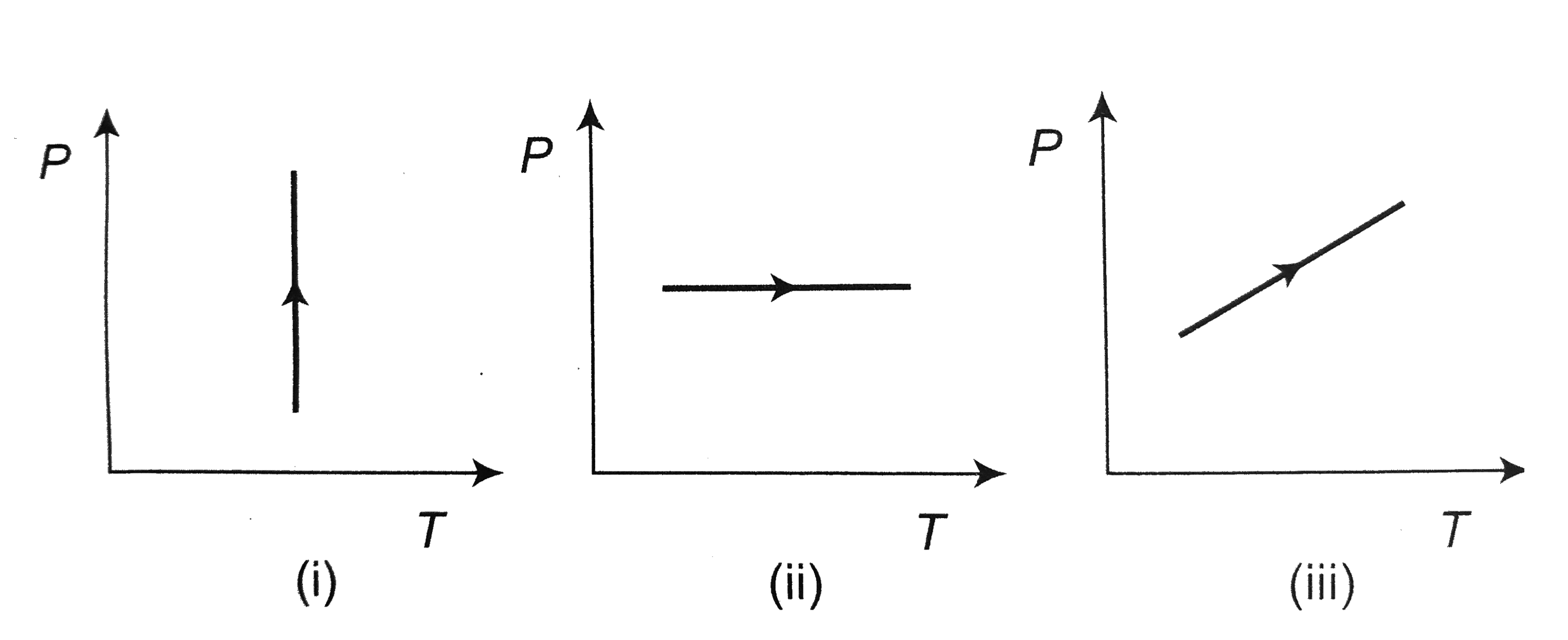
- Pressure versus temperature graphs of an ideal gas are as shown in fig...
Text Solution
|
- In a process the density of a gas remains constant. If the temperature...
Text Solution
|
- The expansion of unit mass of a perfect gas at constant pressure is sh...
Text Solution
|
- Air is filled at 60^(@)C in a vessel of open mouth. The vessle is heat...
Text Solution
|
- One mole of an ideal gas undergoes a process p=(p(0))/(1+((V(0))/(V))...
Text Solution
|
- Two identical glass bulbs are interconnected by a thin glass tube. A g...
Text Solution
|
- As shown , a piston chamber pf cross section area A is filled with ide...
Text Solution
|
- A gas has volume V and pressure p. The total translational kinetic ene...
Text Solution
|
- A mixture of n(1) moles of monoatomic gas and n(2) moles of diatomic g...
Text Solution
|
- Four containers are filled with monoatomic ideal gases. For each conta...
Text Solution
|
- 10^(23) molecules of a gas strike a target of area 1 m^(2) at angle 45...
Text Solution
|
- From the following V-T diagram we can conclude:-
Text Solution
|
- The density in grams per litre of ethylene (C(2)H(4)) at STP is :-
Text Solution
|
- A gas is expanded from volume V(0) = 2V(0) under three different proce...
Text Solution
|
- Some of the thermodynamic parameters are state variables while some ar...
Text Solution
|
- For an ideal gas PT^(11) = constant then volume expansion coefficient ...
Text Solution
|
- The internal energy of a gas is given by U= 5 + 2PV. It expands from V...
Text Solution
|
- When water is heated from 0^(@)C to 4^(@)C and C(P) and C(V) are its s...
Text Solution
|
_E01_043_S01.png)