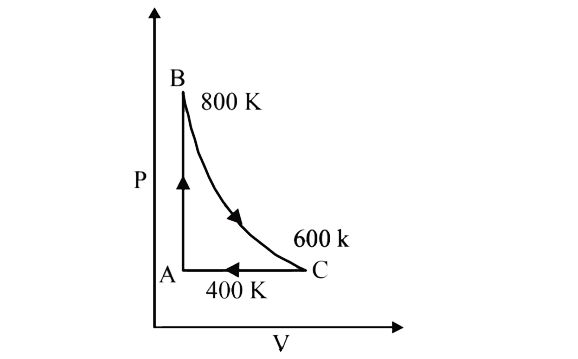
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-GEOMETRICAL OPTICS-EXERCISE - 05 (A)
- A carnot engine operating between temperatures T(1) and T(2) has effic...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- Three rods of Copper, Brass and Steel are welded together to from a Y ...
Text Solution
|
- A solid body of constant heat capacity 1J//^@C is being heated by keep...
Text Solution
|
- Consider a spherical shell of radius R at temperature T. The black bod...
Text Solution
|
- Consider an ideal gas confined in an isolated closed chamber. As the g...
Text Solution
|
- The P - V diagram of 2 gm of helium gas for a certain process A rarr B...
Text Solution
|
- In an ideal gas at temperature T , the average force that a molecule a...
Text Solution
|
- An experiment takes 10 minutes to raise the temperature of water in a ...
Text Solution
|
- using euipartion of energy, the specific heat ("in" jkg^(-1)K^(-1)of a...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- An ideal gas under goes a quasi static, reversible process in which it...
Text Solution
|
- A pendulum clock loses 12s a day if the temperature is 40^@C and gains...
Text Solution
|
- 200 g of water is heated from 40^(@)C "to" 60^(@)C . Ignoring the slig...
Text Solution
|
- The ratio of work done by an ideal diatomic gas to the heat supplied b...
Text Solution
|
- Which of the following shown the correct relationship between the pres...
Text Solution
|
- A carbot freezer takes heat from water at 0^(@)C inside it and rejects...
Text Solution
|