A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-THERMODYNAMICS-Exercise 1
- A diatomic ideal gas initially at 273 K is given 100 cal heat due ...
Text Solution
|
- For an ideal monoatic gas during any process T= kV, find out the...
Text Solution
|
- The maximum efficiency of a heat engine operating between 100 ^(@...
Text Solution
|
- A heat engine operating n between 227^(@) and 27^(@) C absorbs 2 ...
Text Solution
|
- A reversible heat engine A(based on carnot cycle ) absorbs heat fro...
Text Solution
|
- The entropy change when two of ideal monoatomic gas is heated form 200...
Text Solution
|
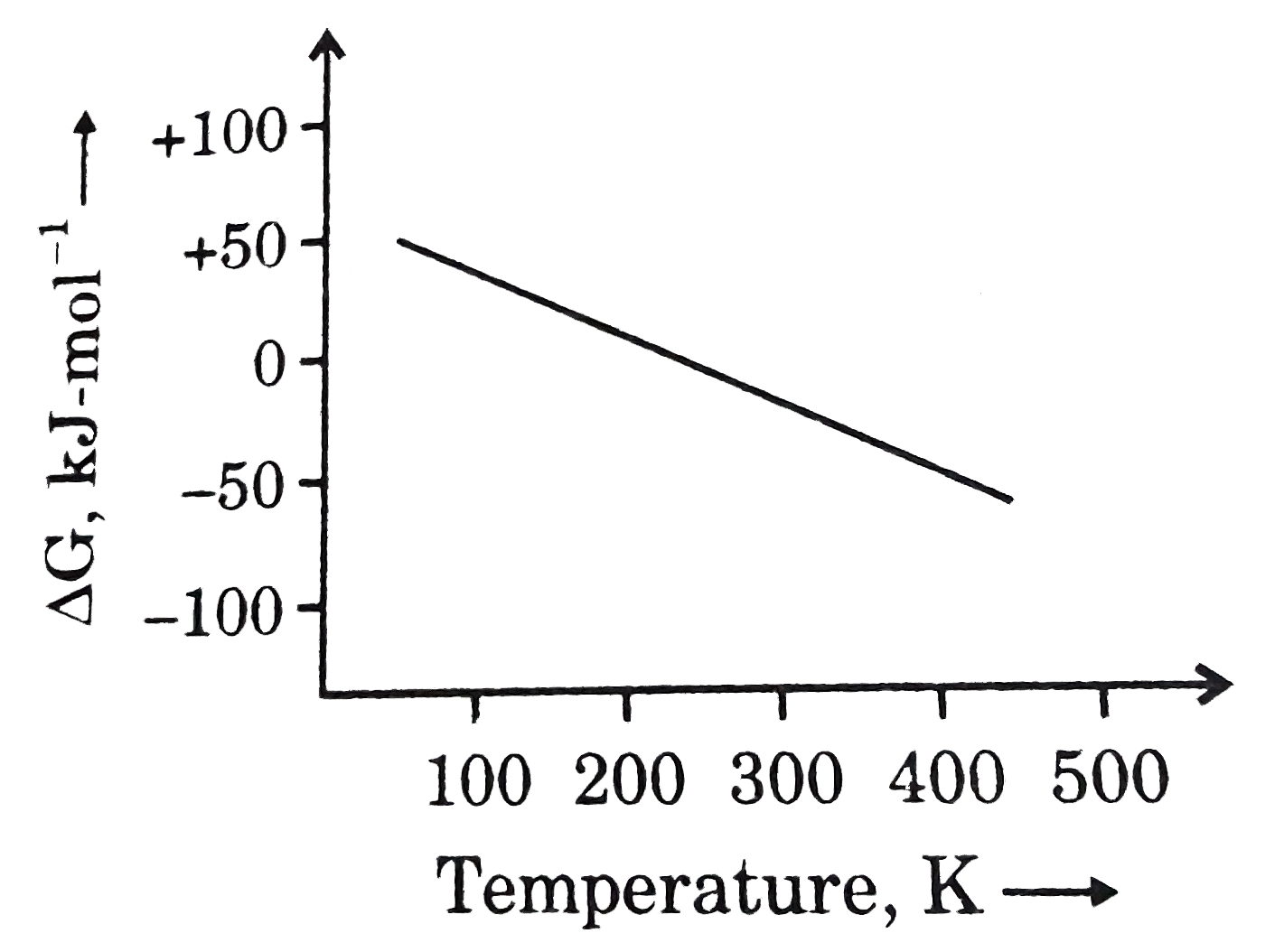
- What can be concluded about the values of DeltaH and DeltaS from this ...
Text Solution
|
- If DeltaH("vaporisation") of substance X (l) (molar mass :30 g/mol) is...
Text Solution
|
- The change in entrophy of 2 moles of ideal gas upon isothermal exp...
Text Solution
|
- Pressure of 10 moles of an ideal gas is changed from 2 atm to 1 at aga...
Text Solution
|
- The enthalpy of tetramerization of X in gas phase (4 X(g) to X(4)(g)...
Text Solution
|
- Standard enthorpy of X(2), Y(2) and XY(3) are 60,40 and 50 JK^(-1...
Text Solution
|
- For the f reaction at 300K A(g)+B(g) to C(g) DeltaU = - ...
Text Solution
|
- The correct relationship between free energy change in a reaction an...
Text Solution
|
- The value of DeltaG(f)^(@) of gaseous mercury is 31 K J //"mole". A...
Text Solution
|
- What is Delta(r)G(KJ/mol) for sysnthesis of ammonia at 298K at follow...
Text Solution
|
- For the reaction takes places at certain tempreature NH(4) HS(s) hArr...
Text Solution
|
- Calculate log(10){[C](eq)//[A](eq)} where [C] and [A] are equilirium ...
Text Solution
|
- What is the free energy charge (DeltaG) when 1.0 mole of water at 100...
Text Solution
|
- What is the free energy charge (DeltaG) when 1.0n mole of water at...
Text Solution
|