A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
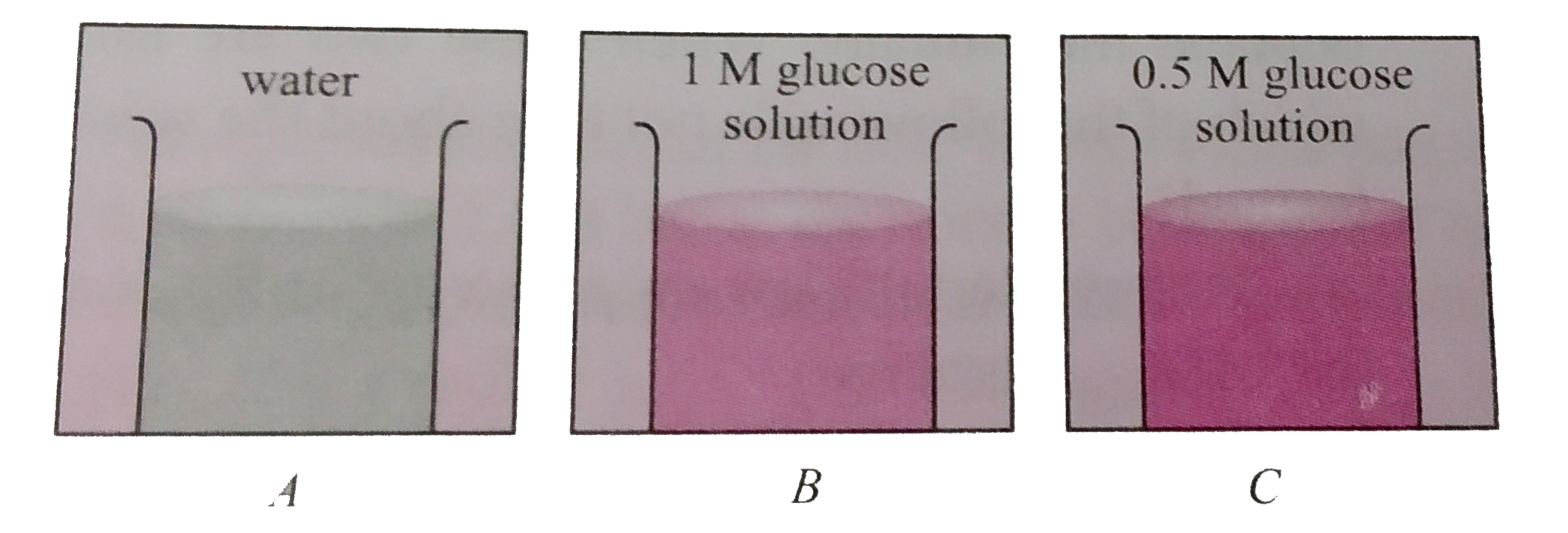
- In three beakers labelled as (A),(B) and (C ) , 100 mL of water, 100 m...
Text Solution
|
- Which has maximum osmotic pressure at temperature T? a. 100 mL of 1...
Text Solution
|
- Mixture containing 100 mL of 1 M urea solution and 300 mL of 1M glucos...
Text Solution
|
- Find the pH of solution prepared by mixing 25 ml of a 0.5 M solution o...
Text Solution
|
- For converting a solution if 100 ml KCl of 0.4 M concentration into a ...
Text Solution
|
- In three beakers labelled as (A),(B) and (C ) , 100 mL of water, 100 m...
Text Solution
|
- To 100 ml of a 0.5 M solution, 400 ml of water is added, the final mol...
Text Solution
|
- 9.0 ग्राम ग्लूकोज को 100 ग्राम जल में घोला गया है। जल का घनत्व 1 ग्राम...
Text Solution
|
- A buffer solution is prepared by mixing 20 ml of 0.1 M CH3COOH and 40 ...
Text Solution
|