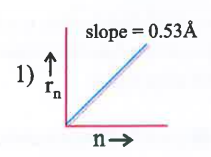
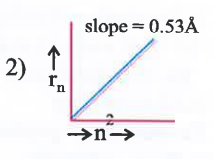
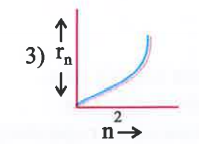
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC STRUCTURE-All Questions
- As the orbit number increase , the distance between two consecutive or...
Text Solution
|
- The ratio of the radius of the Bohr orbit for the electron orbiting th...
Text Solution
|
- Which of the following curves may represent the radius of orbit (r(n))...
Text Solution
|
- How much energy is required to ionise a H atom if the electron occupie...
Text Solution
|
- If the speed of electron in the first bohr orbit of hydrogen atom is x...
Text Solution
|
- If the following mater travel with equal velocity the longest wavele...
Text Solution
|
- Calculate the momentum of radiation of wavelength 0.33 nm
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- In a main energy level, the orbital with more number of nodal planes w...
Text Solution
|
- Choose the correct statement among the following:
Text Solution
|
- The maximum probability of finding electron in the d(xy) orbital is -
Text Solution
|
- Which of the following statements regarding an orbital are correct
Text Solution
|
- Which of the following statements on the atomic wave function psi is n...
Text Solution
|
- What is the full degeneracy of the n =3 state of a H-atom in the absen...
Text Solution
|
- For the azimuthal quantum number 'I' the total number of magnetic quan...
Text Solution
|
- How many sets of four quantum numbers are possible for electron presen...
Text Solution
|
- The set of quantum numbers, n = 2, l = 2, m(l) = 0 :
Text Solution
|
- The sub-energy level which can accommodate maximum number of electrons...
Text Solution
|
- The azimuthal quantum number and the principal quantum number of the 1...
Text Solution
|
- The orbital with lowest energy in which an electron with Azimuthal qua...
Text Solution
|