A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC STRUCTURE-All Questions
- The ratio of slopes of K(max) vs. V and V(0) vs. v curves in the photo...
Text Solution
|
- Photoelectron emission is observed for three different metals A,B and ...
Text Solution
|
- The follwing diagram indicates the energy levels of a certain atom whe...
Text Solution
|
- Which of the following postulates does not belong to Bohr's model of t...
Text Solution
|
- The mass of an electron is m, charge is e and it is accelerated from r...
Text Solution
|
- In two individual hydrogen atoms electrons move around the nucleus in ...
Text Solution
|
- The difference in angular momentum associated with electron in two suc...
Text Solution
|
- The ionization energy of a hydrogen atom in terms of Rydberg constant ...
Text Solution
|
- If the wavelength of series limit of Lyman series for He^(+) ions is x...
Text Solution
|
- The potential energy of an electron in the hydrogen atom is -6.8 eV. I...
Text Solution
|
- What is the potential energy of an electron present in N- shell of th...
Text Solution
|
- The distance between 4th and 3rd Bohr orbits of He^(+) is :
Text Solution
|
- The ratio of velocity of the electron in the third and fifth orbit of ...
Text Solution
|
- If in Bohr's model, for unielectronic atom, time period of revolution ...
Text Solution
|
- The ionization potential for the electron in the ground state of the h...
Text Solution
|
- The mass of a proton is 1836 times more than the mass of an electron. ...
Text Solution
|
- The energy of an electron moving in n^(th) Bohr's orbit of an element ...
Text Solution
|
- Potential energy of electron present in He^(+) is:
Text Solution
|
- The velocity of an e in excited state of H-atom is 1.093 xx 10^(6)m//s...
Text Solution
|
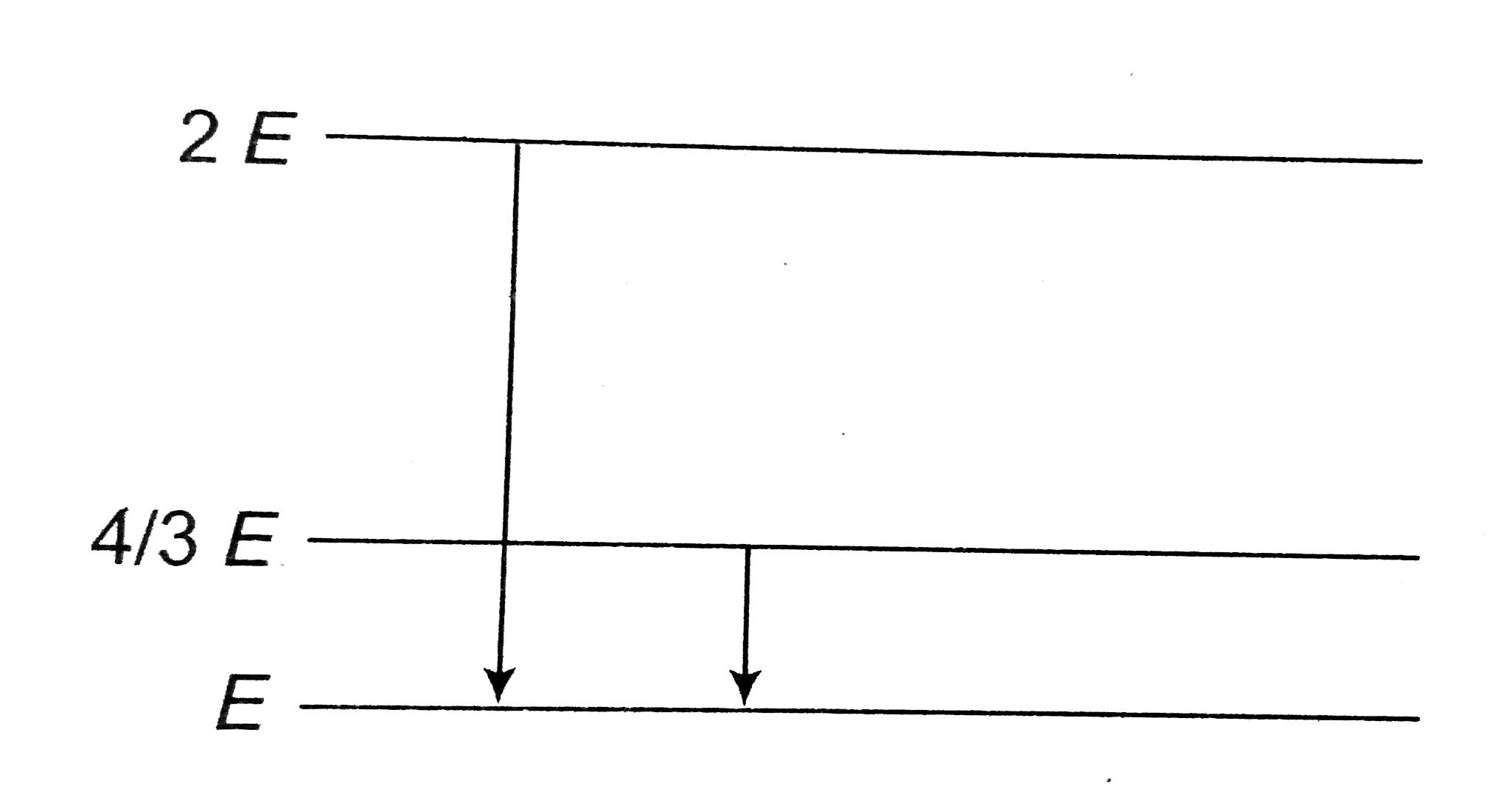
- The energy of a I,II and III energy levels of a certain atom are E,(4E...
Text Solution
|