A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC STRUCTURE-All Questions
- The mass of a particle is 10^(-10)g and its radius is 2xx10^(-4)cm. If...
Text Solution
|
- Which of the following graphs correctly represents the variation of pa...
Text Solution
|
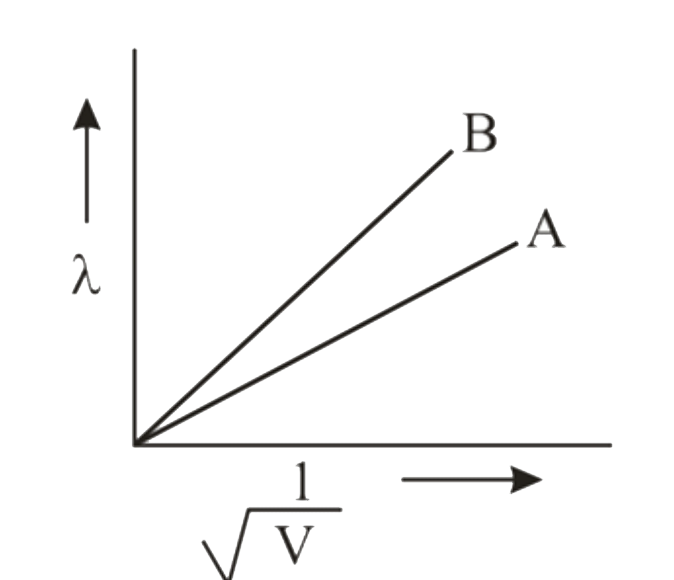
- de Broglie wavelengths of two particles A and B are plotted against (1...
Text Solution
|
- A proton and an alpha particle are accelerated through the same potent...
Text Solution
|
- The ratio of orbital angular momentum and spin angular momentum of an ...
Text Solution
|
- Probability of finding the electron psi^(2) of 's' orbital does not de...
Text Solution
|
- The orbital angular momentum of an electron in 2s-orbital is
Text Solution
|
- The subshell that arises after f is called the g subshell.How many ele...
Text Solution
|
- The quantum numbers of most energetic electron in Ar atom when it is i...
Text Solution
|
- For a 'd' electron, the orbital angular momentum is (h =(h)/(2pi))
Text Solution
|
- The quatum numbers +(1)/(2) and -(1)/(2) for the electron spin represe...
Text Solution
|
- The Schrodinger wave equation for hydrogen atom is Psi(2s) = (1)/(4sqr...
Text Solution
|
- For a 'f' electron the orbital angular momentum is
Text Solution
|
- Magnetic moments of V(Z = 23), Cr(Z = 24), Mn (Z = 25) are x,y,z. Henc...
Text Solution
|
- The value of the magnetic moment of a particular ion is 2.83 Bohr magn...
Text Solution
|
- If nitrogen atoms had el,ectonic configuration is ? It would have en...
Text Solution
|
- If the subsidiary quantum number of a subenergy level is 4, the maximu...
Text Solution
|
- The orbital diagram in which both Pauli's exclusion principle and Hund...
Text Solution
|
- When alpha particle are sent through a thin metal foil ,most of the...
Text Solution
|
- Many elements have non-integral atomic masses because
Text Solution
|