Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC STRUCTURE-All Questions
- The sum of spins of all the electron is the total spins(S) and (2S+1) ...
Text Solution
|
- Spin multiplicity of Nitrogen atom is
Text Solution
|
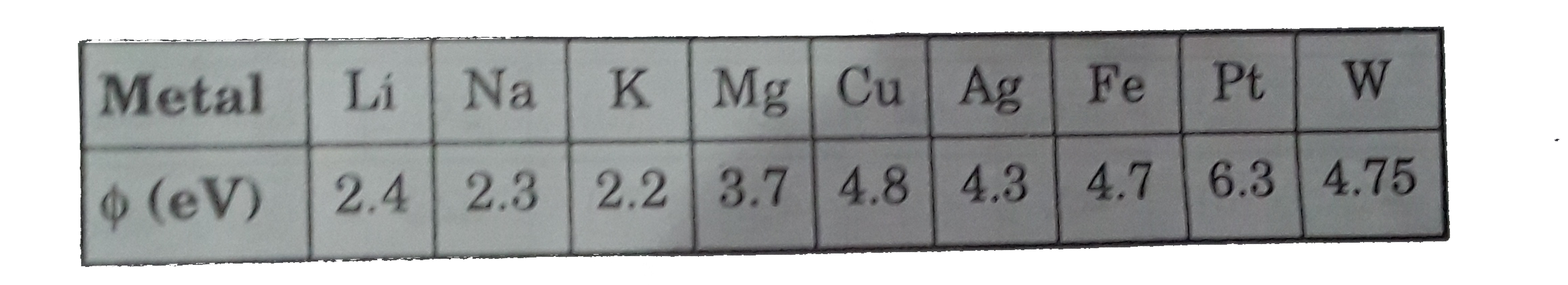
- The work function (phi) of some metals is listed below . The number of...
Text Solution
|
- Difference between n^(th) and (n+1)^(th) Bohr's radius of H atom is eq...
Text Solution
|
- A single electron system has ionisation energy 11180 KJ "mole"^(-1). T...
Text Solution
|
- The number of spectral lines produced when an electron jumps from 5^(t...
Text Solution
|
- In a collection of H-atoms, all the electrons jump from n=5 to ground ...
Text Solution
|
- In a single isolated atom an electron make transition from 5th excited...
Text Solution
|
- The number of waves made by a Bohr electron in Hydrogen atom in one co...
Text Solution
|
- The minimum number of waves made by an electron moving in an orbit hav...
Text Solution
|
- The wave function of an orbital is represented as psi(4,2,0). The azim...
Text Solution
|
- The radial distribution curve of the-orbital with double dumbbell shap...
Text Solution
|
- A compound of vanadium possesses a magnetic moment of 1.73BM. The oxid...
Text Solution
|
- Magnetic moment of M^(x+) is sqrt(24)BM. The number of unpaired electr...
Text Solution
|
- How many d-electrons in Cu^(+)(At.No =29) can have the spin quantum (-...
Text Solution
|
- The maximum number of electrons can have pricipal quantum number n = 3...
Text Solution
|
- 1 mol of photons each of frequency 250s^(-1) would have approximately ...
Text Solution
|
- {:(Column I,Column II),((A)psi(310),(p)5f),((B)psi(120),(q)3p(x) or 3p...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),"Thomson model of atom",(P),"Electrons...
Text Solution
|
- {:(Column I,Column II),((A)"Radial function" psi((r)),(p)"Principle" Q...
Text Solution
|