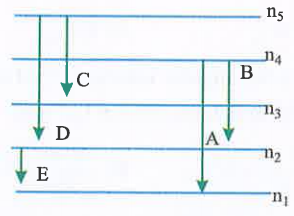
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC STRUCTURE-All Questions
- When an electron makes a transition from (n+1) state of nth state, the...
Text Solution
|
- Monochromatic radiation of wavelength lambda is incident on a hydrogen...
Text Solution
|
- For a hypothetical H like atom which follows Bohr's model, some spectr...
Text Solution
|
- When an electron makes a transition from (n + 1) state to n state ...
Text Solution
|
- Light from a discharge tube containing H-atoms in some excited state, ...
Text Solution
|
- Be^(3+) and a proton are accelerated by the same potential, their de-...
Text Solution
|
- The mass of an electron is m, charge is e and it is accelerated form r...
Text Solution
|
- An electron is continuously accelerated in a vacuum tube by applying p...
Text Solution
|
- An electron of mass m when accelerated through a potential difference ...
Text Solution
|
- The ratio for the gap between successive orbits from the nucleous onwa...
Text Solution
|
- For a 3s - orbital, value of Phi is given by following realation: Ps...
Text Solution
|
- Energy required to ionise 2 mole of gaseous He^(+) ion present in its ...
Text Solution
|
- Condider the following plots for 2s-orbital: x,y and z are respecti...
Text Solution
|
- If n and l are respectively the principal and azimuthal quantum number...
Text Solution
|
- Consider the following six electronic configurations (remaining inner ...
Text Solution
|
- The above electronic configuration deviates from (I) Hund's rule (II...
Text Solution
|
- The ratio of magnetic moments of Fe(III) and Co(II) is:
Text Solution
|
- Give the correct order of initials T(true)F(false) for following satem...
Text Solution
|
- Which of the following statements is/are true in the correct of photoe...
Text Solution
|
- Which is correct graph for photoelectric effect.
Text Solution
|