A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMIC STRUCTURE-All Questions
- Statement-1: Photon has definite momentum though it has no rest mass. ...
Text Solution
|
- Statement-1: The orbital angular momentum of d-electron in orbitals is...
Text Solution
|
- STATEMENT-1: The ground state electronic configuration of introgen is ...
Text Solution
|
- When electron jumps from higher orbit to lower orbit, then energy is r...
Text Solution
|
- When electron jumps from higher orbit to lower orbit, then energy is r...
Text Solution
|
- When electron jumps from higher orbit to lower orbit, then energy is r...
Text Solution
|
- When electron jumps from higher orbit to lower orbit, then energy is r...
Text Solution
|
- When electron jumps from higher orbit to lower orbit, then energy is r...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|
- Werner Heisenberg considered the limits of how precisely we can measur...
Text Solution
|
- The French physicist Louis de Brogie in 1924 postulated that matter, l...
Text Solution
|
- The French physicist Louis de Broglie in 1924 postulated that matter l...
Text Solution
|
- The French physicist Louis de Brogie in 1924 postulated that matter, l...
Text Solution
|
- It is tempting to think that all possible transitions are permissible,...
Text Solution
|
- It is tempting to think that all possible transitions are permissible,...
Text Solution
|
- It is tempting to think that all possible transitions are permissible,...
Text Solution
|
- The Schrodinger wave equation for H-atom is nabla^(2) Psi = (8pi^(2)...
Text Solution
|
- The Schrodinger wave equation for H-atom is nabla^(2) Psi = (8pi^(2)...
Text Solution
|
- The Schrodinger wave equation for H-atom is nabla^(2) Psi = (8pi^(2)...
Text Solution
|
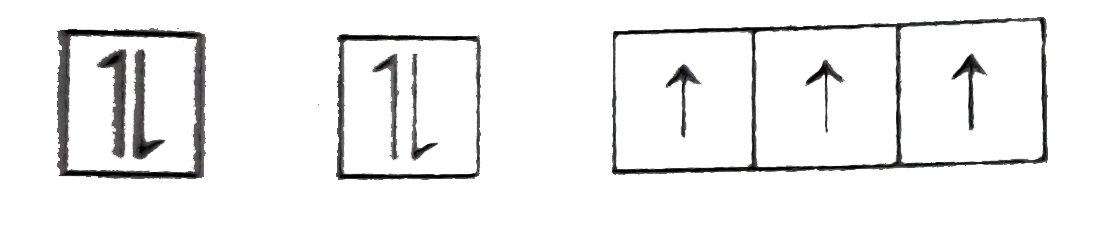 STATEMENT-2: Electronic are filled in orbitals as per aufbau principle, Hund's rule of maximum spin multiplicity and puli's principle.
STATEMENT-2: Electronic are filled in orbitals as per aufbau principle, Hund's rule of maximum spin multiplicity and puli's principle.