A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Statement-1: For the reaction: Ni^(2+)+2e^(-)rarrNi and Fe^(2+)+2e^(...
Text Solution
|
- A fuel cell is a cell that is continously supplied with an oxidant and...
Text Solution
|
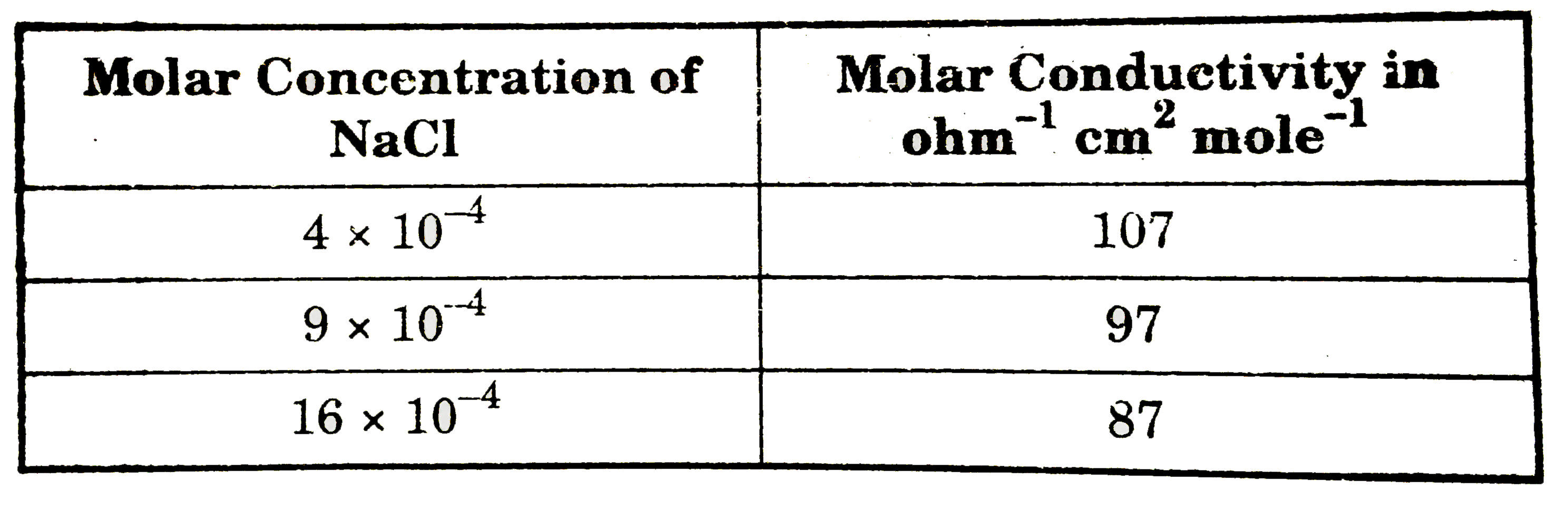
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- The molar conductance of NaCl varies with the concentration as shown i...
Text Solution
|
- Given : E(cu^(+2)//cu)^0=0.34 V E(Cl2//Cl^(-))^0=1.36 V E(Br2//Br^...
Text Solution
|
- The E(Call)^(@)=1.18V for Zn(s)||Zn^(+2)(1M)||Cu^(+2)(1M)|Cu(s). The v...
Text Solution
|
- A fuell cell uses CH(4)(g) and forms CO(3)^(2-) at the anode. It is us...
Text Solution
|
- For the cells in opposition, Zn(s) | ZnCl(2)(sol).|AgCl(s)|Ag|AgCl(s...
Text Solution
|
- The conductivity ofa solution may be taken to be directly proportional...
Text Solution
|
- At 0.04 M concentration, the molar conductivity of solution of an elec...
Text Solution
|
- Calculate [H^+],[HCOO^(-)] and [OCN^(-)] in a solution that contains 0...
Text Solution
|
- To prepare a buffer solution of pH=4.04, amount of Barium acetate to b...
Text Solution
|
- Find the DeltapH( initial pH - final pH) when 100 ml 0.01M HCl is adde...
Text Solution
|
- The ionisation constant of benzoic acid (PhCOOH) is 6.46 xx 10^(-5) an...
Text Solution
|
- When 100 mL of 0.1 M NaCN solution is titrated with 0.1 M HCl solution...
Text Solution
|
- The indicator constant for an acidic indicator, HIn is 5xx10^(-6)M. Th...
Text Solution
|
- Ionisation constant of HA (weak acid) and BOH (weak base) are 3.0xx10^...
Text Solution
|
- pOH=7-0.5pKa+0.5 pKb is true for aqueous solution containing which pai...
Text Solution
|
- An acid-base indicator which is a weak acid has a pK(In) value =5.45. ...
Text Solution
|
- A buffer solution is made by mixing a weak acid HA (K(a) =10^(-6)) wit...
Text Solution
|