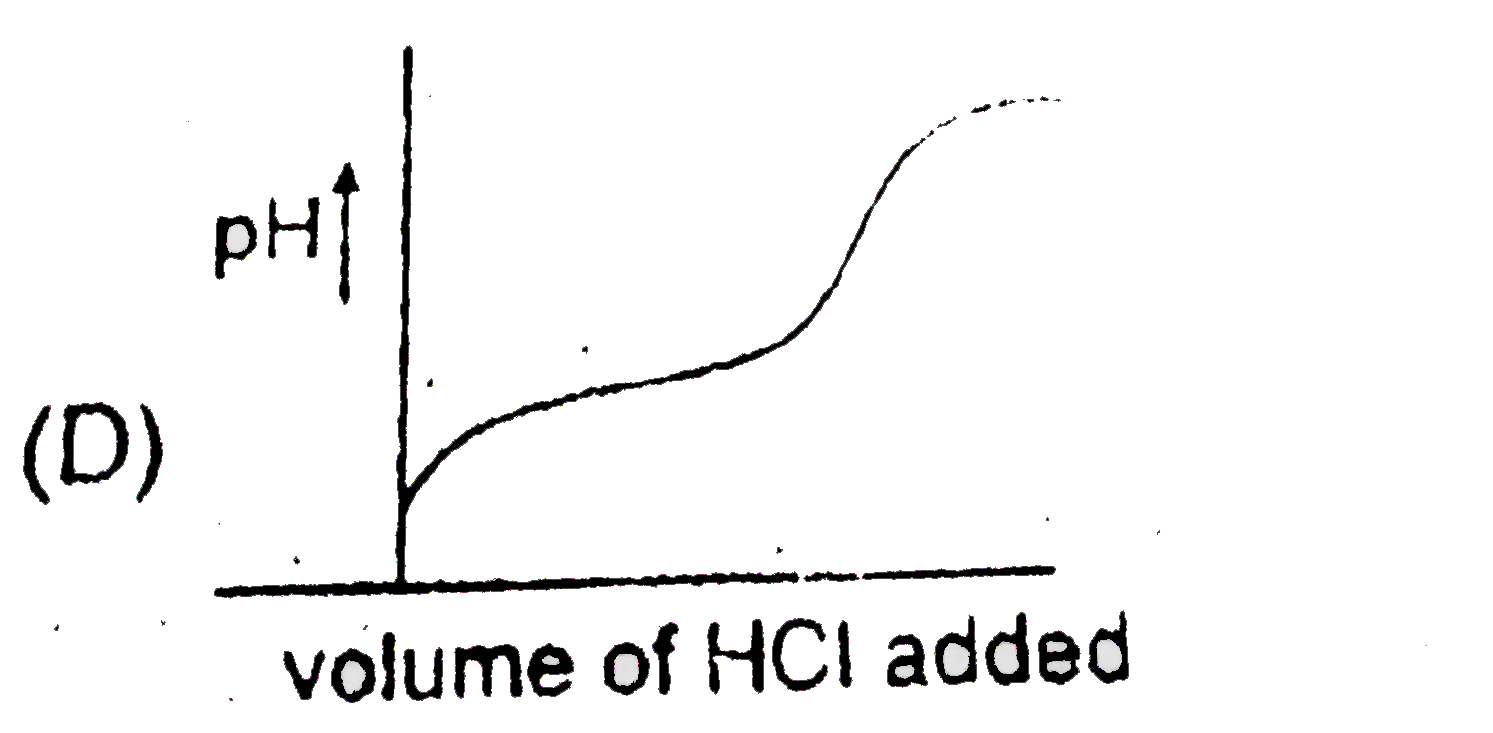
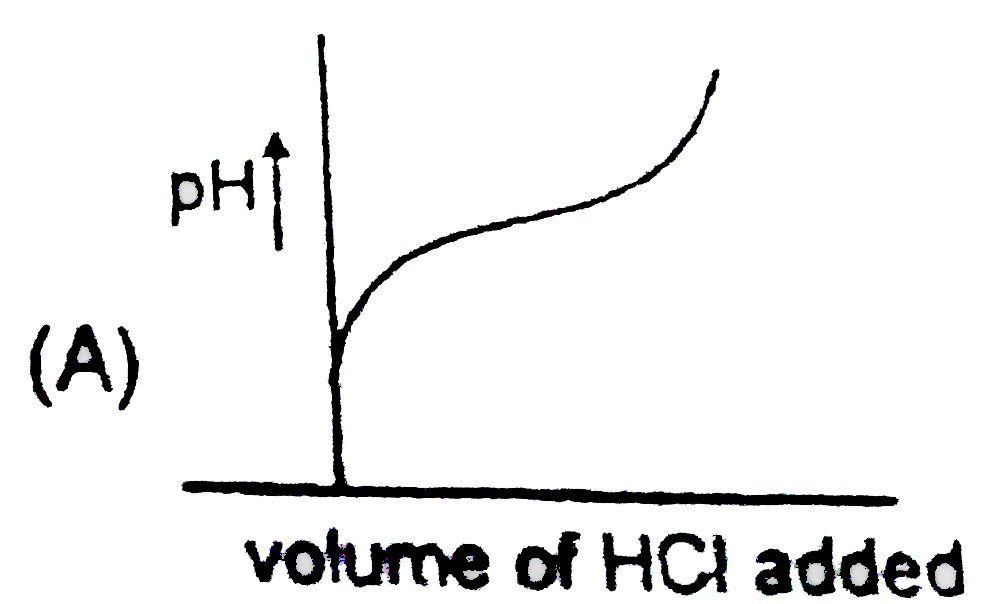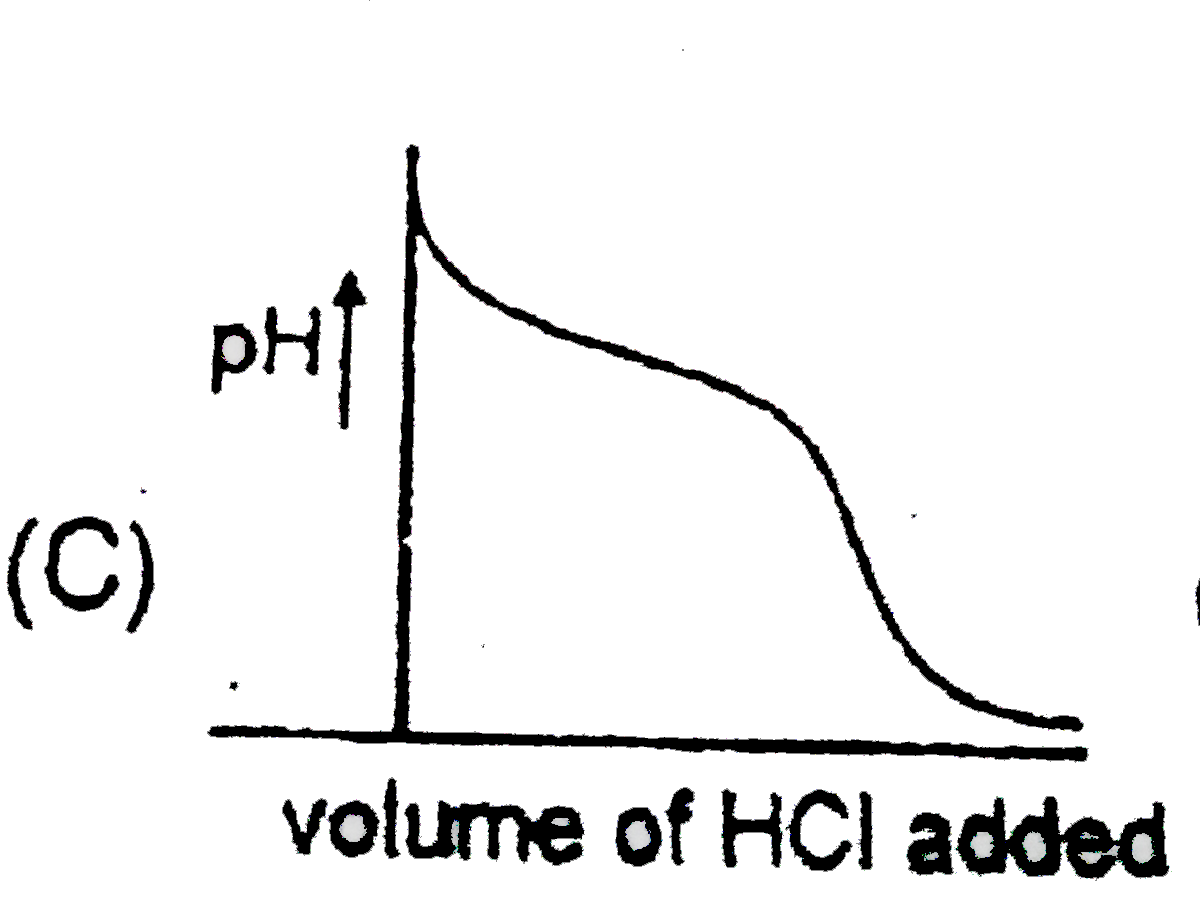A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Find the DeltapH( initial pH - final pH) when 100 ml 0.01M HCl is adde...
Text Solution
|
- The ionisation constant of benzoic acid (PhCOOH) is 6.46 xx 10^(-5) an...
Text Solution
|
- When 100 mL of 0.1 M NaCN solution is titrated with 0.1 M HCl solution...
Text Solution
|
- The indicator constant for an acidic indicator, HIn is 5xx10^(-6)M. Th...
Text Solution
|
- Ionisation constant of HA (weak acid) and BOH (weak base) are 3.0xx10^...
Text Solution
|
- pOH=7-0.5pKa+0.5 pKb is true for aqueous solution containing which pai...
Text Solution
|
- An acid-base indicator which is a weak acid has a pK(In) value =5.45. ...
Text Solution
|
- A buffer solution is made by mixing a weak acid HA (K(a) =10^(-6)) wit...
Text Solution
|
- Which of the following solutions when added to 1L of a 0.1 M CH3COOH o...
Text Solution
|
- Select the correct statements:
Text Solution
|
- Buffer soution'A' of a weak monoprotic acid and it's sodium salt in co...
Text Solution
|
- Statement-1 : 0.20 M solution of NaCN is more basic than 0.20 M soluti...
Text Solution
|
- Assertion : A substance that can either act as an acid a base is calle...
Text Solution
|
- Consider a solution of CH3COONH4 which is a salt weak acid and weak ba...
Text Solution
|
- Consider a solution of CH3COONH4 which is a salt weak acid and weak ba...
Text Solution
|
- Consider a solution of CH3COONH4 which is a salt weak acid and weak ba...
Text Solution
|
- [H^(+)] concentration in 0.01 M H2O2 solution (K(a(1))=3xx10^(-12) and...
Text Solution
|
- Calculate the hydrogen ion concentration ("in mol"//dm^3) in a solutio...
Text Solution
|
- The vapour pressure of the solution of two liquids A(P^@=80mm)and B(P^...
Text Solution
|
- Osomotic pressure [in atm] of a 0.1 M solution of K4[Fe(CN)6], which u...
Text Solution
|