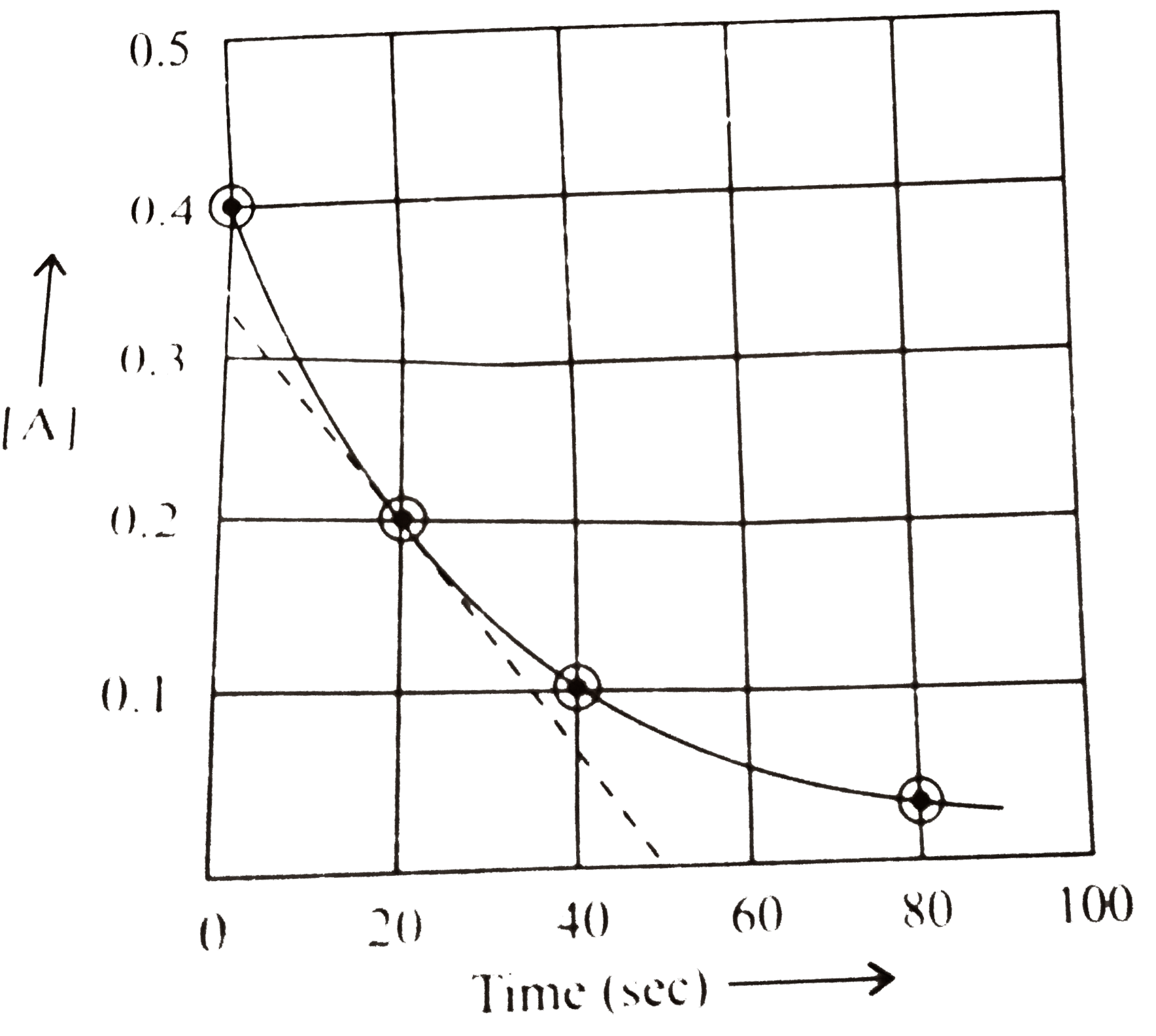
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- The substance undergoes first order decomposition.The decomposition fo...
Text Solution
|
- A certain reaction obeys the rate equation (in the integrated form) [C...
Text Solution
|
- A certain reaction A rarr B follows the given concentration (Molarity)...
Text Solution
|
- Consider the following case of competing 1^(st) order reactions. ...
Text Solution
|
- Assertion (A) : If the activation energy of a reaction is zero, temper...
Text Solution
|
- The rate law expresses the relationship of the rate of a reaction to t...
Text Solution
|
- The rate law expresses the relationship of the rate of a reaction to t...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- The following data were observed for the following reaction at 25^(@)C...
Text Solution
|
- Match the following columns
Text Solution
|
- Match order of the reaction (in List-I) with the corresponding rate co...
Text Solution
|
- For the reaction Ato prouducts, the following data is given for a part...
Text Solution
|
- If (dx)/(dt)=k[H(3)O^(+)]n and rate becomes 100 times when pH change...
Text Solution
|
- The gas phase decomposition of dimethyl ether follows first order kine...
Text Solution
|
- 105 mL of pure water at 4^(circ)C saturated with NH(3) gas yielded a s...
Text Solution
|
- Volume V(1)mL of 0.1 M K(2)Cr(2)O(7) is needed for complete oxidation ...
Text Solution
|
- 100 ml of 0.1 M NaAl(OH)(2)CO3 is neutralised by 0.25 N HCl to form Na...
Text Solution
|
- 25mL of 2N HCl, 50 mL "of" 4N HNO(3) and xmL H(2)SO(4) are mixed toget...
Text Solution
|
- An excess of NaOH was added to 100 mL of a ferric chloride solution.Th...
Text Solution
|