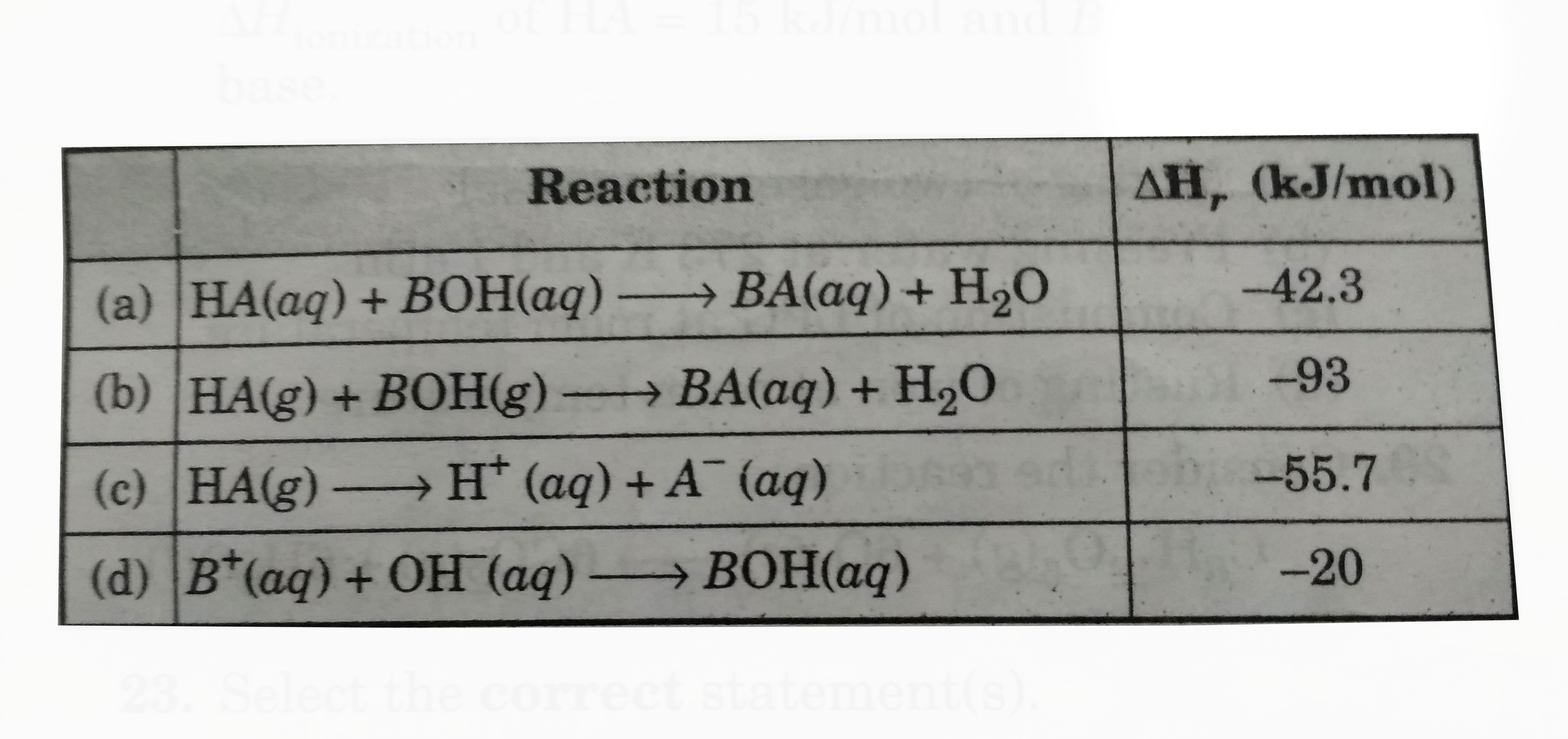
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Consider the following statements and arrange in the order of true/fa...
Text Solution
|
- One mole of an ideal diatomic gas (C(v) =5 cal) was transformed from i...
Text Solution
|
- From the following date , mark the opation(s) where Delta H is correct...
Text Solution
|
- Statement-1: Due to adiabatic free expansion temperature of real gas m...
Text Solution
|
- Statement-1 :When a gas at high pressure expands against vacuum, the m...
Text Solution
|
- Staetement -1: The magniyude of the work involed in an isothermal expa...
Text Solution
|
- Assertion(A) : Heat of neutralisation of HCl and NaOH is same as that ...
Text Solution
|
- Statement -1 in the following reaction : C(s)+O(2)(g)to CO(2) (g), D...
Text Solution
|
- {:(,"Column-I",,"Column-II"),((a),"Reversible adiabatic compression",(...
Text Solution
|
- {:(,"Column-I",,"Column-II"),((a),C ("s,graphite")+0(2)(g)rarrCO(2)(g)...
Text Solution
|
- Calculate the pH at which the following reaction will be at equilibriu...
Text Solution
|
- In an ionic solid r((+)) = 1.6 Å and r((-)) = 1.864 Å. Use the radius ...
Text Solution
|
- A crystal is made up of particals X, Y, and Z. X froms fcc packing. Y ...
Text Solution
|
- Diamond has face-centred cubic lattice. There are two atoms at (0, 0, ...
Text Solution
|
- In hexagonal close packing of spherer in three dimensions.
Text Solution
|
- If the anions (A) form hexagonal closest packing and cations (C) occup...
Text Solution
|
- Analysis show that nickel oxide consists of nickel ion with 96% ions h...
Text Solution
|
- In an fcc crystal, which of the following shaded planes contain the gi...
Text Solution
|
- Following three planes (P(1), P(2), P(3)) in an fcc unit cell are show...
Text Solution
|
- In a AB unit cell (Rock salt type) assuming A^(+) forming fcc :
Text Solution
|