A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Analysis show that nickel oxide consists of nickel ion with 96% ions h...
Text Solution
|
- In an fcc crystal, which of the following shaded planes contain the gi...
Text Solution
|
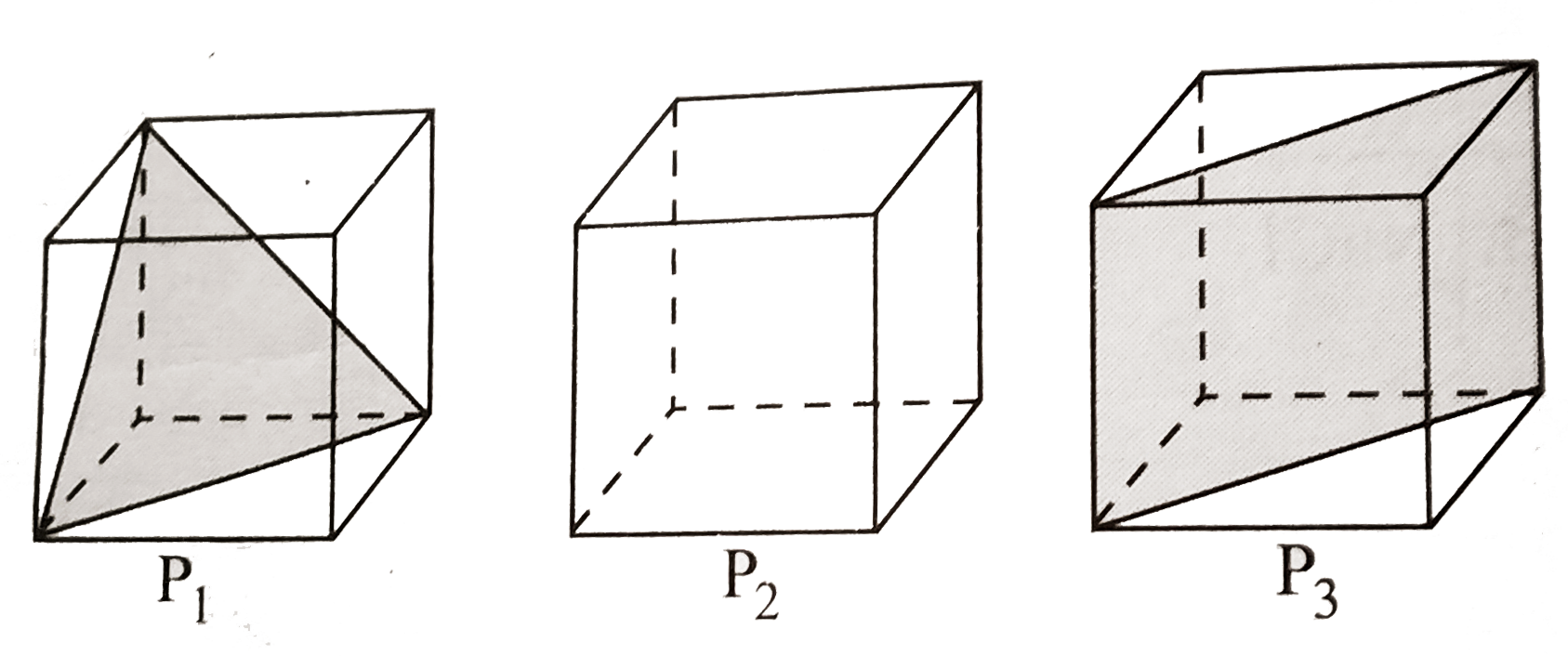
- Following three planes (P(1), P(2), P(3)) in an fcc unit cell are show...
Text Solution
|
- In a AB unit cell (Rock salt type) assuming A^(+) forming fcc :
Text Solution
|
- In the fluorite structure if the radius ratio is (sqrt(3)/(2)-1) how m...
Text Solution
|
- The co-ordination number of FCC structure for metals is 12, since:
Text Solution
|
- An hcp and a ccp structure for a given element would be expected to ha...
Text Solution
|
- Assertion : An important feature of fluorite structure is that cations...
Text Solution
|
- Assertion :In NaCIcrystal each Na^(+) ion is tourching 6 CI^(-) ion bu...
Text Solution
|
- When an atom or an ion is missing from its nomal lattice site a lattic...
Text Solution
|
- Column-I and Column-II contains four entries each.Entries of Column-I ...
Text Solution
|
- A(2)B molecules(" molar mass " = 259.8 g//"ml") crystallises in a hex...
Text Solution
|
- In F.C.C. arrangement of identical spheres, distance between two neare...
Text Solution
|
- N2 and H2 are taken in 1:3 molar ratio in a closed vessel to attained ...
Text Solution
|
- The unit of equilibrium constant KC of a reaction is "mol"^(-2) boat^2...
Text Solution
|
- NH4COONH2 (s) hArr 2NH3(g)+CO(2)(g) If equilibrium pressure is 6 atm...
Text Solution
|
- A 10 litre box contains O3 and O2 at equilibrium at 2000 K. Kp=4xx10^(...
Text Solution
|
- The following two reactions: i. PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) ...
Text Solution
|
- For the chemical reaction 3X(g)+Y(g) hArr X(3)Y(g), the amount of ...
Text Solution
|
- What is the minimum mass of CaCO3(s), below which it decomposes comple...
Text Solution
|