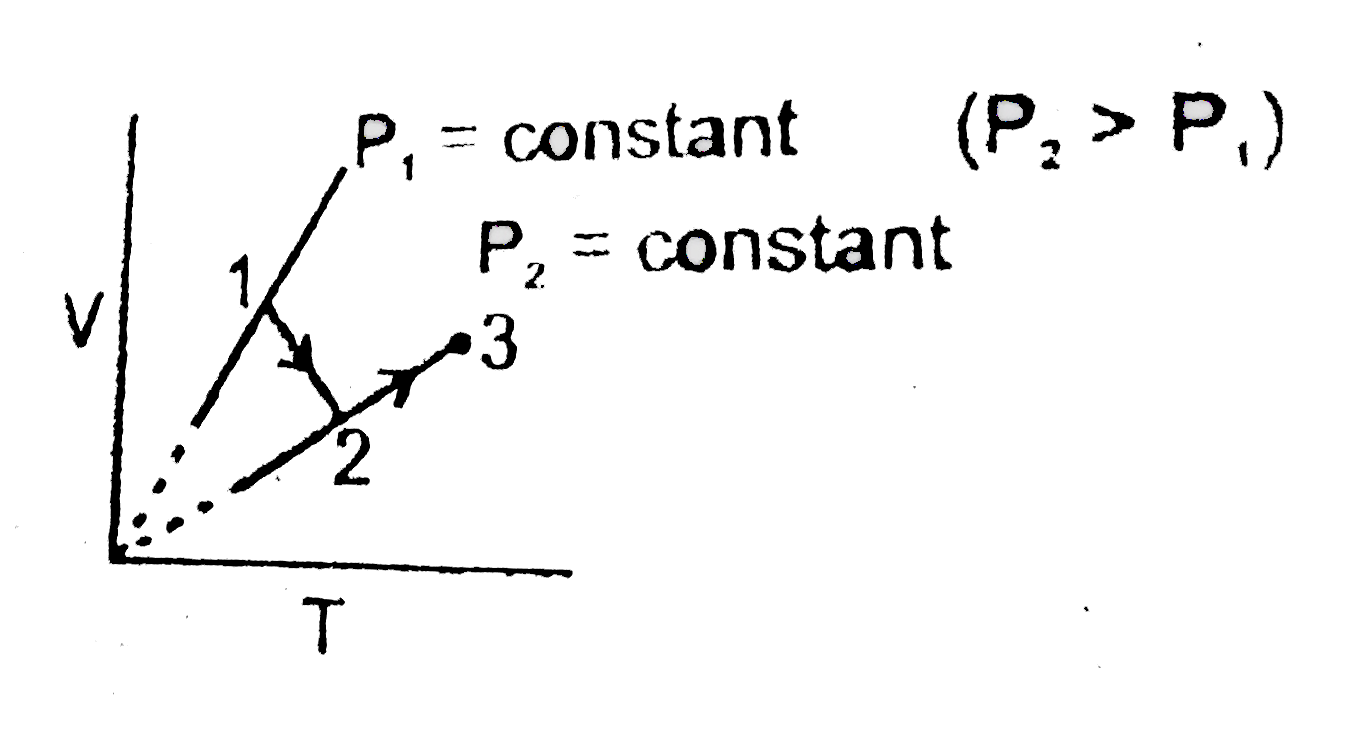
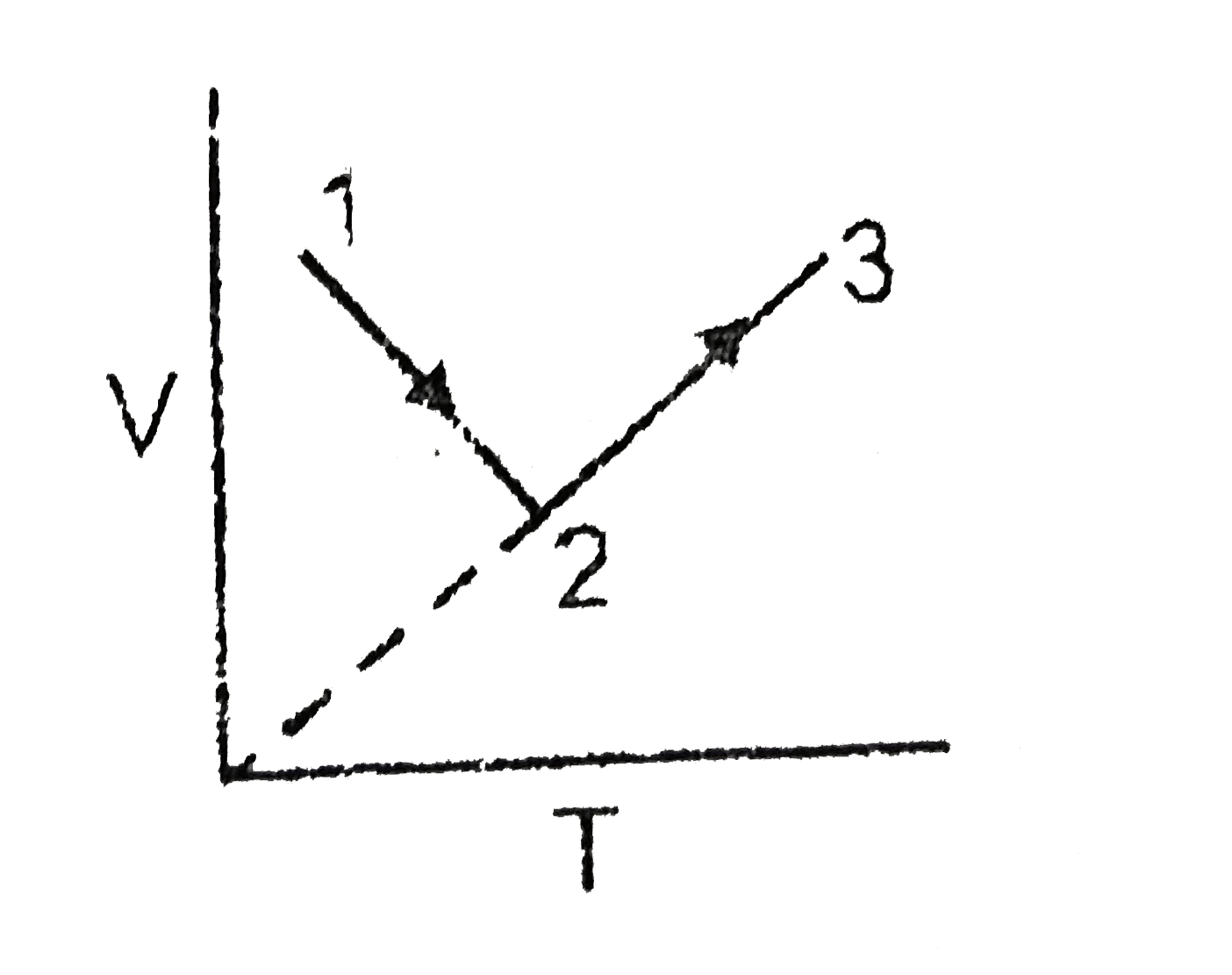A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Consider the following statements and arrange in the order of true/fal...
Text Solution
|
- If the pressure of the gas contained in a closed vessel is increased b...
Text Solution
|
- Following graph is constucted for the fixed amount of the gas
Text Solution
|
- A gaseous organic compound has a density of 2.5 kg//m^3 at 2 atm and a...
Text Solution
|
- Two flask A and B have equla volumes.Flask A contains hydrogen at 600 ...
Text Solution
|
- Assertion : The value of van der Waal's constant a is larger for ammon...
Text Solution
|
- Assertion: In a container containing gas 'A' at temperature 400 K, som...
Text Solution
|
- Statement-1 :The area under the maxwell distribution molecular speed c...
Text Solution
|
- Single option match maxtix : {:("Column-I","Column-II"),((A)(Vm-b)(P...
Text Solution
|
- {:(,"Coloumn-I",,"Column-II"),("(a)","At low pressure",,(p)Z=1+(pb)/(R...
Text Solution
|
- A bulb of constant volume is attached to a manometer tube open at othe...
Text Solution
|
- The pressure exerted by 12 g of an ideal gas at temperature t^(@)C in ...
Text Solution
|
- A 4 : 1 mixture of helium and methane contained in a vessel at 10 bar ...
Text Solution
|
- The number of photons emitted in 10 hours by a 60 W sodium lamp (lambd...
Text Solution
|
- The De-broglie wavelength of a tennis ball of mass 66 g moving with th...
Text Solution
|
- The photon emitted due to electronic transition of 5^(th) excited stat...
Text Solution
|
- A proton when accelerated through a potential difference of V volt has...
Text Solution
|
- The ionization energy of a hydrogen atom in terms of Rydberg constant ...
Text Solution
|
- Number of electron having l+m value equal to zero in .(26)Fe may be
Text Solution
|
- The ration of the e//m (specific charge) values of an electron and an ...
Text Solution
|